INTRODUCTION
Research teams increasingly use social media to supplement nondigital methods for recruiting research participants. This trend is likely to grow as recruitment continues to pose a major challenge to clinical trials and other forms of human subject research. The European Patients’ Academy (EUPATI) defines clinical trials as research studies involving people (healthy volunteers or patients) that test the safety and efficacy of a new medical treatment, device, surgical procedure, or medical test. Without these volunteers, this type of research and medical progress would be impossible; however, research participants can be hard to recruit. A recent systematic review found that 76.1% (131/172) of randomised clinical trials were discontinued due to poor recruitment.1
Social media provides a new gateway for connecting larger and hard-to-reach groups of the population with research opportunities. Social media includes widely accessible web-based and mobile information tools which allow users to view, create, and share information with others online.2 The ability to build a network, i.e., social networking, makes social media unique and distinguishes these platforms from other interactive websites such as Craigslist (Craigslist, Inc., San Francisco, California, USA) or Wikipedia, (Wikimedia, San Francisco, California, USA) for example. While 56% of Europeans were using social media in 2018,3 the use of this media varies across European countries and globally, as shown in Figure 1 and Table 1. The most popular platforms among Europeans include Facebook (Facebook Inc., Menlo Park, California, USA), Pinterest (Pinterest, Inc., San Francisco, California, USA), Twitter (San Francisco, California, USA), YouTube (Google, San Bruno, California, United States), Instagram (Facebook Inc., Menlo Park, California, USA), Tumblr (Verizon Media, New York City, New York, USA).
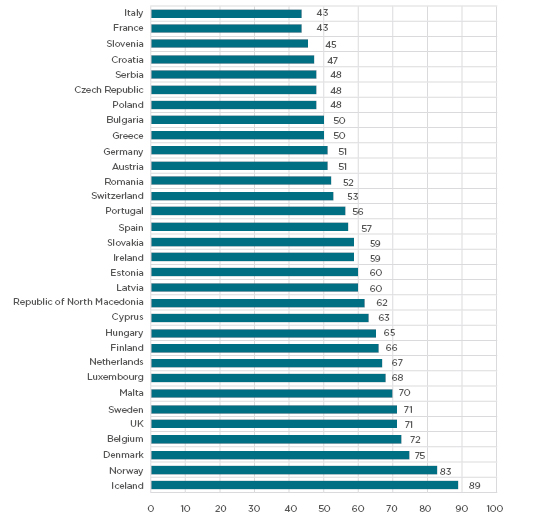
Figure 1: Social media use among adults in European countries in 2017.
Adapted from StatCounter Global Stats.3
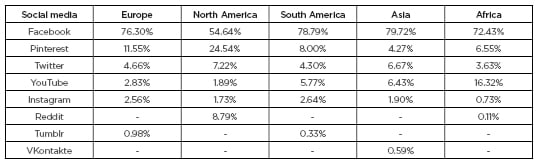
Table 1: Use of social media in Europe, North America, South America, and Asia for May 2019.
The table only includes the top eight social media platforms used. Social media are ranked according to the amount of traffic they refer to other websites indicating usage.
Adapted from StatCounter Global Stats.3
The ability to target segments of the population based on their demographic characteristics, interests, and previous online activities differentiates digital media, such as social media, from other more traditional recruitment approaches via printed flyers or mailed postcards, community talks, billboards, newspapers, TV, or radio advertisements (ads). Through paid ads, social media platforms, such as Facebook, allow the targeting of users with specific characteristics, e.g., age, sex, location, language, race and ethnicity, interests, and behaviours. Trial recruitment messages are presented to these audiences over a couple of days to reach a certain number of impressions, i.e., times a post is displayed to users regardless of whether the post is clicked or not, and to ultimately get users to click on the message link, view a webpage, and take action. In addition to paid ads, social media also allows users to send nonpaid, organic messages that may be seen by followers of an account or people who are online at the same time or interested in the same topic. Paid and organic messages provide the link to the study page. On a clinical study webpage, users might be asked to complete a prescreening survey, contact the study team, or provide consent and download a mobile health intervention. However, not every social media platform permits paid advertising of clinical trials; it is prohibited on Twitter and Pinterest, and requires prior authorisation on other platforms such as Facebook, Instagram, YouTube, and Reddit (Advance Publications, San Francisco, California, USA). Despite these restrictions, social media provides new opportunities for identifying and engaging with potential clinical trial participants including previously under-represented groups, such as women and some minorities. So, is it worthwhile for research teams to invest limited time and resources in social media-based recruitment?
CAN SOCIAL MEDIA-ENABLED RECRUITMENT MOVE THE NEEDLE?
Although researchers have reported mixed results after using social media for research participant recruitment, there is some initial evidence of its efficiency and effectiveness,4,5 i.e., the European Physical Activity through Sustainable Transport Approaches (PASTA) project.6 The study team concluded that Facebook and Twitter served as “time-efficient” venues to recruit 17.39% (1,859/10,691) participants from seven European cities in a longitudinal, web-based survey. In some cases, social media offered an even more cost-effective solution. Guthrie et al.7 used Facebook to recruit postmenopausal women, aged 45–70 years, with bothersome vulvovaginal symptoms within 20 miles of the study sites in Minneapolis, Minnesota, or Seattle, Washington, USA, for a randomised, double-blind, placebo-controlled trial. Over 28 days, they enrolled 8.3% (25/302) of their trial participants via Facebook at the cost of $14,813, compared to 277 women who they recruited via 277,000 direct recruitment mailings at the expense of $98,682.
For the most part, social media has complemented, rather than replaced, traditional recruitment efforts, but it has the potential to accelerate the timeline toward achieving the accrual target. For example, in a randomised controlled trial in the UK to assess the effectiveness of a behavioural intervention and prevent weight gain over the Christmas holiday period, the study team used social media to recruit 11% (35/311) of their adult participants aged 18 years and older with a BMI ≥20.8 However, other recruitment methods were equally important and included word-of-mouth, recruitment at workplaces, community events, and schools.
It may be less of a surprise that social media has shown to be a useful tool to connect research opportunities with younger audiences as reported by Wisk et al.9 The team used Facebook, Twitter, and Instagram to recruit college students aged 17–25 years with Type 1 diabetes mellitus onto an online longitudinal intervention trial. They achieved a retention rate of 88.4%. What about mid-life and older patients in the population, however? There are also encouraging reports of success. Langbaum et al.10 were able to enrol 75,351 cognitively healthy adults aged 55–75 years in USA into a registry (GeneMatch) of Alzheimer’s disease prevention studies online, over half of whom (60%) joined the registry based on social media ads. A second study by Nash et al.11 reported dwindling participant recruitment at 20 months and showed a significant increase in recruitment of middle-to-older-aged people into a blood pressure randomised controlled trial after implementing a 4-month Facebook ad campaign. The team was able to increase the average number of recruited study participants from 1.8 per month using conventional recruitment to 7.3 per month using Facebook (p<0.05).
Finally, one might believe that it is harder to recruit healthy research volunteers, i.e., as a control group, because they have no vested interest in research outcomes related to a specific disease. However, recruitment of healthy elderly participants aged ≥60 years for a Phase I clinical trial on Facebook showed, within a reasonably short period of 8 weeks, that a total of 621 people responded to Facebook ads of whom 45 (7.25%) enrolled.12
These examples demonstrate the potential of social media that is already being harnessed to support clinical study recruitment. While most study teams report the use of paid social media ads, primarily on Facebook, some groups were also successful in recruiting participants using nonpaid organic social media-based approaches. Adrian et al.13 used ‘friend requests’ on Facebook to recruit 66.7% (212/318) young adults who were previously involved in health research into a new mental health study. Another experimental approach that is being tested consists of monitoring public Twitter conversations for targeted recruitment.14 The idea is to engage and recruit those patients in clinical trials who have already discussed their disease experience on Twitter. However, most studies reported the use of social media for study recruitment outside Europe with the USA, Canada, and Australia among the leading countries. Hence, a unique opportunity presents itself to better the understanding of social media as a tool for enhancing clinical trial recruitment within and across different countries and populations in Europe.
BARRIERS THAT AFFECT THE BROADER APPLICATION AND ASSESSMENT OF SOCIAL MEDIA-ENABLED RECRUITMENT
While some studies offer early signs of promise supporting the use of social media as a cost-effective recruitment method, more research is required to determine how best to use social media for research recruitment. For example, is social media better suited for the recruitment of study participants from certain demographic groups or groups with specific health conditions? What are the characteristics of effective messaging approaches? What are social media users’ attitudes on different platforms toward using social media data and digital ad techniques for clinical trial recruitment, or how does the type of research question affect the suitability for social media-based recruitment?
Additionally, some barriers prevent the broader use and thorough assessment of social media-based recruitment in clinical and human subjects research (Box 1), e.g., digital media offers us the possibility to measure recruitment efforts in much more robust ways. However, the collection and reporting of data about digital forms of recruitment are still in its infancy. Guidelines for reporting the design, implementation, and results of social media-enabled recruitment methods are needed to allow the learning from existing recruitment strategies and the development of better ones. The lack thereof hampers the development of evidence-based recruitment methods and the ability to reliably compare the effectiveness of recruitment efforts across different social media platforms, disease trials, and populations. Another challenge is posed by the lack of clear guidance to assist researchers and institutional review board members with the use of social media in human subject research in general and clinical research participant recruitment, which affects both investigator-initiated or sponsored research. Concerns may include how to engage with patients on social media directly, how to manage and respond to user comments, how to track and report adverse events shared by users on social media, or how to avoid introducing potential biases within a study. The lack of consistent regulatory guidelines makes it especially challenging to leverage social media-based recruitment for multi-site trials.
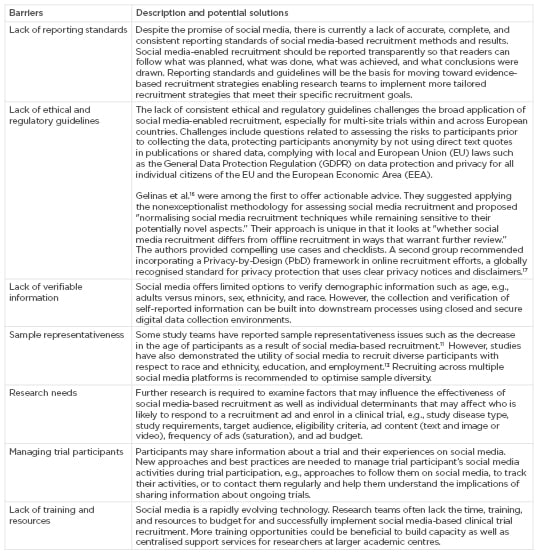
Box 1: Examples of barriers to the broader application and assessment of social media-enabled recruitment, and potential solutions.
Notably, the use of social media targeting capabilities based on users’ previous behaviour, e.g., what they searched for, liked, or mentioned on social media, raises new types of user and data privacy issues and may be perceived as “creepiness”16 by members of the institutional review board, researchers, and social media users alike. While this is an area that requires more research, Gelinas et al.16 were among the first to offer actionable advice in this regard. As they put it: “it is doubtful that the mere perception of creepiness has intrinsic ethical weight or would demand greater protection for social media users.” Instead, they suggested applying the nonexceptionalist methodology for normalising social media-based recruitment techniques while remaining sensitive to new aspects. More practically, this means comparing social media with commonly used, more traditional approaches. In the case of social media-based targeting, one could question whether targeting individual women with young infants via customised ads on Facebook gives rise to greater research and privacy risks compared with the targeting of women with young infants generally at a paediatric office. After all, the personal information on which digital ads are based is usually not available to research teams; it is part of a proprietary algorithm owned by the website or ad company. That said, researchers have the obligation to familiarise themselves with the privacy policy and terms of use of social media to assess whether the proposed research adheres to a website’s terms of service and to ensure that the site will not use the data it collects, from tracking responses to recruitment ads, in ways that violate ethical norms and regulations. Finally, social media is only the first step toward enrolling study participants. If the enrolment rate is considered one of the primary outcomes for assessing the effectiveness of social media-based recruitment, it is vital to pay equal attention to improving downstream recruitment and retention processes such as follow-up, screening, consent, treatment adherence, and data collection. Such steps in the clinical research recruitment funnel need to be optimised, i.e., through chat bots, also known as conversational agents, and artificial intelligence-driven software programs designed to interact with potential study participants or media-rich electronic consent forms and teleconsent15 that reduce the complexity and inaccessibility of common consent forms. Box 1 lists additional barriers that may hinder the application and assessment of social media-based recruitment and possible solutions.
CONCLUSION
The potential in social media for enhancing clinical trial recruitment is real. However, are we harnessing its full potential? The answer is no. More proactive and collective research efforts by European countries to address some of the concerns and barriers could help to leverage the full potential of social media for clinical trial recruitment. The question is also not solely if social media is more effective or cost-efficient than traditional recruitment methods. It will require a combination of the best traditional and digital recruitment methods to address the clinical trial recruitment challenge in new ways.








