Abstract
Rare diseases are individually rare but collectively common, with a combined prevalence of 3.5–5.9%. A common feature of many diseases is a substantial delay in patients receiving a correct diagnosis; this protracted path to diagnosis is termed ‘the diagnostic odyssey’. During the COVID-19 pandemic, significant concerns have emerged from both clinicians and patients regarding a disproportionate effect of the pandemic on diagnosis and management of rare disease. Such concerns prompted a study to explore this question further, the results of which are presented here.
A cross-sector multi-stakeholder coalition was formed, Action for Rare Disease Empowerment (ARDEnt), with representation from patients with rare diseases and carers, patient advocacy groups, clinicians, academics, data scientists, and industry. A mixed methods approach was used to collect and collate information about the impact of the pandemic on diagnostic delay in rare disease. Currently, there is a lack of systematic recording and reporting of rare disease diagnosis in the UK, which created challenges in directly measuring diagnosis rates. Therefore, the group was dependent on a mix of data sources to reflect healthcare provided during 2020 compared with previous years. The findings were synthesised to describe the impact of the pandemic along the path to diagnosis, from the moment of first concern and engagement with health services, to the availability of definitive testing.
In conclusion, evidence suggests the pandemic has exacerbated the problem of diagnostic delay for rare diseases, affecting all points on the path to diagnosis. The authors recommend three actions to help address this: optimising remote clinical consultations; enhancing the use of health informatics in rare diseases; and proactively identifying patients with undiagnosed rare diseases missed due to the pandemic. This study also highlights the need for better reporting of rare disease diagnoses, a core metric to measure the impact of health system changes that may be put into place to address the priorities of The UK Rare Diseases Framework, also published this year.
Key Points
1. Evidence suggests the COVID-19 pandemic disproportionately exacerbated diagnostic delay for rare diseases, affecting all points on the path to diagnosis.2. To capture the impact and opportunities for the rare disease community, a cross-sector multi-stakeholder coalition, Action for Rare Disease Empowerment (ARDEnt), was formed.
3. The group made three key recommendations for mitigating the effect of the pandemic on the ‘diagnostic odyssey’ in rare disease: optimising remote clinical consultations; enhancing the use of health informatics in rare diseases; and proactively identifying patients with undiagnosed rare diseases missed due to the pandemic.
INTRODUCTION
Rare diseases, defined as affecting fewer than 1 in 2,000 individuals,1 are individually rare but collectively common, with an estimated combined prevalence of 3.5%–5.9%.2 Frequently, patients with rare diseases spend years, or even decades, on a path to diagnosis, hence the described ‘diagnostic odyssey’.3,4 Along this path, patients undergo multiple referrals, investigations, often misdiagnoses, and the frustrations of unanswered questions and unaddressed deterioration in their condition. Many patients with rare diseases never receive an accurate diagnosis5,6 (Figure 1).
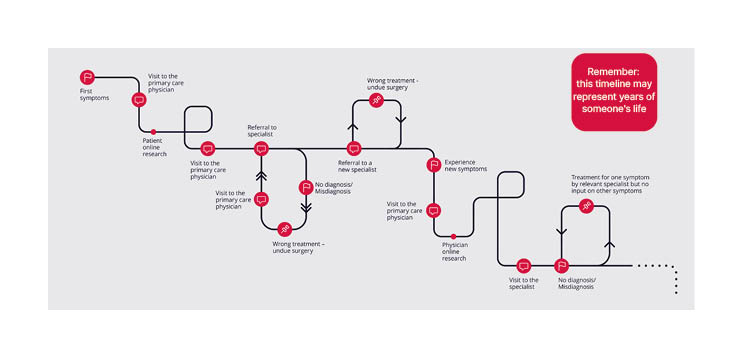
Figure 1: The early stages of the diagnostic odyssey.
The COVID-19 global pandemic has led to significant morbidity and mortality, and a substantial impact on all aspects of life including the provision of health and social care.7 The full extent of the impact continues to emerge. In England there was a substantial drop in primary care consultation rates that only recovered in May 2021, 2 years post the start of the pandemic. This drop was also reflected in the number of secondary care referrals, with numbers in January 2021 still below the 4 year average. The number of diagnoses for a range of chronic conditions also fell significantly. The impact of the pandemic was not experienced evenly across all patient groups. For example, it was widely recognised early in the pandemic that direct morbidity and mortality was greatest in the elderly population; however, indirect health impacts, although harder to capture, were almost certainly more widely spread, with young patients, especially those under 11 years, experiencing the largest fall in consultation rates, and largest drop in number of GP appointments.8
It was recognised early in the global pandemic that the rare disease community may be disproportionately affected. Many rare conditions make those affected more vulnerable to the complications of COVID-19, so shielding and socially isolating oneself was recommended for many. This led to further psychosocial impact on patients and their families due to a profound and prolonged reduction in contact with others and wider society.9 Patients with rare diseases are often young, and are often frequent users of health and social care services; with the pandemic causing disruption of these services, patients with rare diseases were therefore disproportionately affected.10 With reduced access to health services and the halting of some diagnostic services, there was concern that the diagnostic odyssey would be further protracted. In addition, with both pre-clinical and clinical research halted or limited, the opportunity for advances in disease understanding and treatments were curtailed.
To capture the impact and learnings from the COVID-19 pandemic, as well as the potential opportunities that may arise for the rare disease community, a cross-sector multi-stakeholder coalition was formed, Action for Rare Disease Empowerment (ARDEnt). ARDEnt is made up of over 30 individuals and groups, including patients, advocates, healthcare practitioners, industry representatives, scientists, data specialists, and clinical trial organisers. The principal aim of this group was to obtain and collate information in order to inform the Action Plans outlined in the UK Rare Diseases Framework,1 published in January 2021, and to suggest best practice for UK patients with rare diseases post-pandemic. This was successfully published via the ARDEnt report entitled: ‘Making the unseen seen: Rare disease and the lessons learned from the COVID-19 pandemic’.11
METHOD
The ARDEnt group was founded by three patient advocacy group leaders in the rare disease field, who put out an open invitation in April 2020 to rare disease stakeholders to join a meeting regarding the impact of the pandemic on the rare disease community. Additional members were recruited to ensure complete cross-sector representation of stakeholders, and to address specific gaps in knowledge by both snowballing and purposive recruitment. Following initial discussion by the ARDEnt group, three priority themes were identified, under which evidence would be collated based on expert opinion. These themes were agreed to be of critical priority, and were subsequently included in the UK Rare Diseases Framework as three of the four priorities described by The Department of Health and Social Care.
Theme 1: Diagnostic Delay Theme
2: Health and Social Care Coordination Theme
3: Research and Drug Development including Access to Treatment
A mixed methods approach was used to synthesise both quantitative and qualitative data.
Information was collected by the following methods: review of published literature, review of grey literature (including government, patient advocacy, and public health documents), and interviews with key stakeholders (e.g., patients, clinicians, nurse specialists, and representatives of patient advocacy groups).
A literature search was performed in Pubmed and Google Scholar, using the keywords “COVID-19” and synonyms, combining this with the term “Rare disease” and synonyms. Due to the paucity of relevant literature in the initial searches, this search was repeated on further occasions. Additional literature was identified by manually reviewing the references of identified literature. Due to the emerging nature of the pandemic, much of the literature identified was not published in academic journals. The grey literature was identified on the government webpage and patient advocacy group searches, with additional insights and inputs advised by members of the multi-stakeholder ARDEnt team.
The interviews were semi-structured, using a standard questionnaire with open questions. Interviews were transcribed, and quotes were used in the subsequent report. A systematic literature review was not performed, due to a general lack of peer-reviewed literature on the effect of the pandemic on rare disease, and relevant information appearing in a range of sources.
Although the focus of this review was the UK, the information search was not restricted to the UK.
Discussion within the ARDEnt Theme 1 group identified the following sub-themes, under which information would be captured:
Pre-engagement with healthcare
Primary care
Referral to secondary care
Secondary and tertiary care
Diagnostics
These sub-themes reflect the steps in a diagnostic path for a rare disease. Additionally, a specific cohort of patients was identified and examined separately: children aged 0–5 years.12 This cohort was highlighted, following discussion with multiple stakeholders, because 70% of rare diseases present in childhood and rare diseases that present at this age frequently have a time-critical diagnosis, with even modest delay significantly affecting outcome.2
Both quantitative and qualitative data was captured, and the elements synthesised to draw conclusions and recommendations for reducing diagnostic delay in rare disease. A similar approach was used by Theme 2 and Theme 3 of ARDEnt. The group published their findings on all three themes and their recommendations in an online report called: ‘Making The Unseen Seen: Rare disease and the lessons learned from the COVID-19 pandemic’, which has been presented to The Department of Health and Social Care.
RESULTS
Absolute Diagnosis Rate
Because of the challenges in collecting absolute numbers of people diagnosed with a rare disease in a given time period, four rare liver disorders were examined as a proxy. These conditions were chosen as the diagnosis is made as an inpatient and associated with a liver biopsy, enabling robust identification in Hospital Episodic Statistic (HES) data. The average number of diagnoses per month in January–September 2020 showed a reduction of 36% in comparison to the same months in 2019 (Bythell, personal communication), suggesting the pandemic caused an exacerbation of delay.
A specialist nurse recorded new presentations of one rare metabolic condition to specialist services dropping from an average of 13 per year in 2017–2019 to only seven in 2020 (Bell, personal communication), four of whom were diagnosed in January or February.
Patient groups have also noted the decline in the number of newly diagnosed families requesting information and registering for services. One group supporting patients and families affected by rare genetic conditions reported a 33% decline in 2020 compared to 2019. SWAN UK reported a 52% reduction in online registrations in 2020 compared to 2019 (Roberts, personal communication).
International experience has shown similar reductions in rare disease diagnosis. For example, in the Italian region of Campania, rare disease certificates (a record of new diagnoses) reduced from 1,272 in the first 4 months of 2019 to 774 in the same period of 2020.13
Pre-Engagement with Healthcare
‘Stay at home. Protect the NHS. Save lives’14 messaging by the UK Government from March 2020, resulted in four people in 10 feeling too concerned about burdening the NHS to seek help from their GP in April 2020.15 People were confused about what services were still available, and concerned about the danger of going to hospitals. Emergency department attendances reduced by approximately 25% across the UK.16-28 In a survey conducted in April 2020, one-third of paediatricians working in emergency departments or paediatric assessment units witnessed delayed presentations.29
Primary Care
Diagnosis of a rare condition often depends on multiple consultations and a holistic view of the patient, a challenge even before the pandemic. During the pandemic, the number of primary care appointments fell from 6,026,140 in the first week of March 2020, to 4,225,502 in the last week.30 Even in December 2020, although numbers had recovered somewhat, primary care appointments were still 11% below January’s numbers (calculated from data30). Of these, the number of face-to-face consultations declined from 80% before the pandemic to 60%, replaced by telephone and video consultations.31
Referrals to Secondary Care
Referrals to specialist services fell during the pandemic.32-34 Clinical Genetics service referrals in some areas fell by >50% during April–June 2020 in comparison to the same months in 2019 (Menzies, personal correspondence).
Secondary and Tertiary Care
Outpatient appointments, inpatient, and diagnostic services changed significantly, especially during the lockdowns driven by peaks in COVID-19 cases. Services such as Clinical Genetics have looked to assess both the challenges and potential benefits of the increase in telemedicine, including both telephone and video consultations. The Clinical Genetics Society Telemedicine Survey 2020 captured 2,204 responses from clinical genetics doctors, genetic counsellors, and specialist nurses across 13 centres in the UK (Menzies, personal communication). The majority of appointments were being carried out by telephone call, with video being the next most frequent form of consultation. The survey highlighted some of the challenges in maintaining services during the pandemic: adapting the remote service for specific user needs; inability to examine the patient; and difficulty explaining complicated genetic concepts remotely. Advantages of using telemedicine were also identified: patients being in their own environment enabled them to relax, have family members with them, and easier access to personal records. Patients did not need to travel, and this provided a safe solution for those who were self-isolating or shielding (Menzies, personal communication).
Diagnostic Services
There was a significant reduction in activity in the 15 most frequently requested investigations in England, from a mean of 1,967,376.25 per month in 2019 to 1,521,507.17 per month in 2020.35 This was reflected in three of the investigations most commonly used in rare disease diagnostics, those being Echocardiography, Radiology with contrast (including MRI, CT, and non-obstetric ultrasound), and Gastroscopy, according to HES data.36 When comparing the average for April–December 2019 to April–December 2020, there was a 40% reduction of gastroscopies, 30% reduction in echocardiography, and 24% reduction in radiological investigations with contrast.35
Genomic testing, key for most rare diseases, was rationalised with guidance on testing prioritisation issued by NHS England in March 2020.37 Genetic laboratories were instructed to reduce testing to urgent services only, in part to release capacity to support COVID-19 testing. Requests for microarrays, often the first line genetic test for many patients suspected of having an undiagnosed rare disease, substantially reduced (Figure 2).
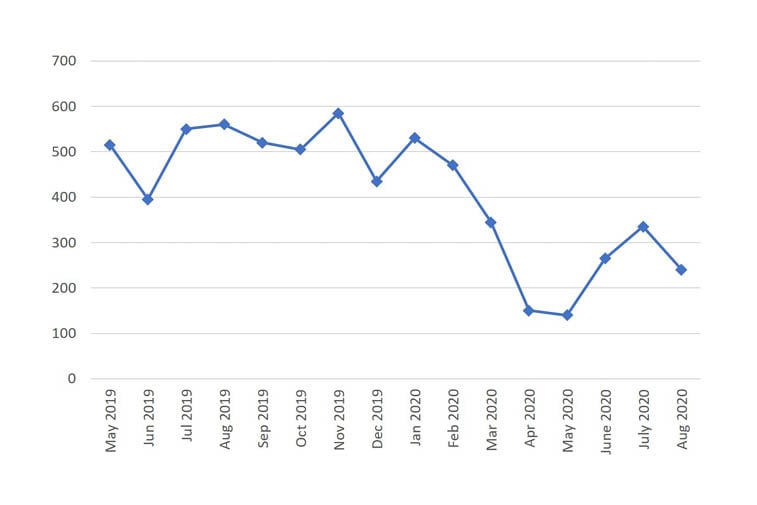
Figure 2: Number of microarray requests over a 16 month period from 2019 to 2020.
Data from an NHS genetics laboratory serving a UK urban population of 5 million people. Reproduced with permission from Menzies, personal communication, 2020.
Children Aged 0–5 Years
70% of rare conditions present in childhood, and 30% of people with a rare condition die before their fifth birthday.1,2 Infant Physical Examinations (one element of the ‘Newborn and Infant Physical Examination’ [NIPE]) were delayed 2 weeks, and performed at single consultations with first immunisations,38 reassuringly without impact on NICE key performance indicators. Data for how 1- and 2-year checks have been impacted during this time period remains unpublished 2 years later. The ‘Babies in Lockdown’ report presented an online survey of 5,474 expectant mothers, parents of infants and toddlers, of whom fewer than one in 10 had seen a health visitor face-to-face in the 103 days of the first lockdown.39 The ‘Working for Babies Report’ identifies “threats to physical health as a result of lockdown, reduced health services and parental reluctance to access them” as a key hidden harm of lockdown on young children.39
Restrictions in social interactions have seriously reduced the number of people young children encounter, including their wider family, social network, and healthcare professionals. This has left parents to oversee their children’s developmental progress without external support. A nurse specialist highlighted the case of an infant who received a diagnosis of a rare metabolic condition during the first national lockdown because their condition was identified by their grandmother. They recognised the enlarged abdomen when changing their nappy as similar to that of their late child who had died 30 years previously. With this knowledge, they demanded that they were seen in A&E urgently, which led to their diagnosis. Had this child not lived with their grandparent, the diagnosis may not have been made.
DISCUSSION
This is the first attempt that the authors are aware of to capture the impact of the pandemic on the path to diagnosis for patients with rare diseases. The breadth of information captured in this report is a reflection of the ARDEnt group, a broad coalition of stakeholders with a broad range of experience and expertise, adding to the value and applicability of the findings and conclusions. The group acknowledges limitations in its methods of sampling including a lack of representation of some voices (e.g., those of young people and ethnic minorities). This reflects a far-reaching need for better diversity and inclusion within rare disease advocacy, necessitating the creation of groups, campaigns, and reports that highlight this particular issue.40-43
Based on the findings, the group makes three recommendations for mitigating the impact of the COVID-19 pandemic on diagnostic delay in rare disease: optimising remote clinical consultations; enhancing the use of health informatics in rare diseases; and proactively identifying patients with undiagnosed rare diseases missed due to the pandemic. These recommendations have been put to The UK Rare Disease Implementation Board for consideration when drawing up the action plans for the UK framework.
The fundamental question, “has the pandemic impacted rare disease diagnosis rates?”, was difficult to clearly answer. The authors used a narrow and defined subset of rare liver disease diagnoses as examples, as these diseases were chosen to ensure confidence in the accuracy of the numbers. The authors’ inability to provide data for a greater number of diseases with the same degree of confidence, an acknowledged limitation of this study, reflects how rare diseases are coded in UK hospital data sets. This limitation in coding had an additional impact on the rare disease community early in the pandemic, when shielding guidance was decided by diagnostic coding in these records. The lack of specificity of rare disease coding led to inappropriate advice given to many patients with rare diseases. This blindspot, the inability to accurately assess the impact on diagnosis, is applicable beyond this specific question of the pandemic, and has relevance when assessing any external impact or intervention on rare disease diagnosis. How will the actions taken to address the first priority of the UK Rare Disease Framework: helping patients get a faster diagnosis, be measured for their impact? Consequently, one of the authors’ three recommendations is to enhance health informatic use in rare diseases. Specifically, they recommend increased resourcing of the National Disease Registration Service (NDRS) to enhance their rare disease scope, and ensuring the standardisation of rare disease clinical coding terms.
Despite this limitation, the authors have collected a range of personal experiences and data that demonstrates a drop in rare disease diagnosis, from initial engagement with health services, referral for investigation or specialist assessment, and registering with patient advocacy groups for support. For example, there was a significant reduction in the number of diagnostic tests such as echocardiograms, radiological investigation, and gastroscopies performed, as well as genetic testing with microarrays. Microarrays are a powerful indicator of the impact on rare disease diagnosis; they are largely requested by secondary care specialists who are not geneticists, and requested as part of the diagnostic workup of patients with developmental delay/intellectual disability, autism spectrum disorder, and multiple congenital anomalies, i.e., patient cohorts that are enriched with undiagnosed rare diseases.
The change in consultation method in primary care, from a largely face-to-face model to a substantially greater use of video or telephone consulting, is also occurring in secondary care, and is perhaps the most significant change in the day-to-day practice of medicine of the pandemic. As of April 2021, primary care still had 41% of all consultations held via video or telephone consulting, an increase of 27% when compared to April 2019.44 Although a welcome adaptation for many patients, and one broadly encouraged to continue, the widespread adoption is balanced by some associated risks. Ensuring that remote consulting is used appropriately is the second recommendation, the optimisation of remote clinical consulting. This includes greater clarification of which type of consultations are appropriate to be performed remotely and which are not, building on the evidence base for what, when, and with whom remote or face-to-face consultation is optimal, with specific attention paid to the risk of exacerbating health inequality. Optimal use of remote consulting also requires that the systems themselves can sustain this way of working. This requires suitable investment in IT platforms, protected times for clinicians, and training to ensure excellent standards of care, and that remote diagnostics are available, with laboratory testing set up to enable remotely-collected samples to be analysed sometime after their collection. Also, there needs to be better interlinking of local and national services, so that information held in electronic health records can be shared and available to those that need it. With suitable investment, game-changing opportunities such as a multidisciplinary team approach for the diagnostic workup of those undiagnosed, but suspected of having a rare disease, could be implemented with members of the multidisciplinary team and the patient remote to one another. This would enable national collaboration and the opportunity to bring the expert to the patient earlier in their diagnostic workup.
The pandemic has led to an increased number of undiagnosed patients with rare diseases. The size of this population is less clear, emphasising the need for suitable investment in the collection of data through organisations such as NDRS, so that the longer term effects of the COVID-19 pandemic on diagnostic delay and resulting outcomes are understood, and systems put in place to mitigate them in future public health emergencies.
It needs to be acknowledge that people living with an undiagnosed rare disease will have been missed. This report highlights that even before engagement with health services, reduced social interaction reduced the number of opportunities for problems to be identified and flagged by extended family members or social circle. The authors’ third recommendation is proactively identifying undiagnosed patients with rare diseases missed due to the pandemic. They suggest a face-to-face developmental assessment for every child aged 0-5 years, who has not been seen face-to-face by services since March 2020. They are asking for a plan on how the inevitable backlog of investigations will be addressed, and evidence to confirm that referral and diagnostic requests are returning to a pre-pandemic level. In order to catch up diagnoses, the authors recommend putting pathways in place to enable much earlier guidance for testing than prior to the pandemic, with the aid of remote consulting where appropriate.
CONCLUSIONS
The COVID-19 pandemic exacerbated the problem of diagnostic delay in rare diseases, and it is likely that the true extent of this delay will not be fully understood for a number of years. The authors identify specific issues and gaps in how the impact of the pandemic on rare disease diagnosis could be assessed, gaps which would be equally applicable to another external event, or an intervention put in place to improve diagnosis. They also highlight some opportunities that have arisen, such as remote consulting, that could improve rare disease diagnosis going forwards. Many of the challenges outlined in the ARDEnt report simply represent exacerbated existing problems. A positive side effect of the pandemic is improved collaboration and cross-sector engagement around how to solve these issues. The three recommendations proposed would help mitigate the lasting impact of the COVID-19 pandemic, and ensure that the lessons learnt will improve rare disease diagnosis.








