Abstract
The remodelling of the heart that occurs after myocardial infarction independently contributes to heart failure (HF) progression. In a rapidly growing patient population with chronic HF, new, safe, and effective therapeutic strategies are needed. Recently, injectable biomaterial-based therapies have been gaining interest, especially for patients post myocardial infarction who have had complications due to advanced HF. One such intervention, based on Algisyl® (LoneStar Heart Inc., Irvine, California, USA) injections, has been examined in the recent prospective, randomised, controlled AUGMENT-HF clinical trial. This paper briefly presents characteristics of Algisyl, an injectable calcium alginate hydrogel; describes a minimally invasive myoplasty procedure for the insertion of Algisyl implants; and provides a concise overview of the design, methods, and results of the AUGMENT-HF study. This paper focusses on the promising findings that Algisyl, in addition to standard medical therapy, was more effective than the standard medical therapy alone for providing sustained 6-month and 1-year benefits in exercise capacity and symptomatic improvement among patients with advanced HF. In addition, this report discusses some implications and challenges relevant to the AUGMENT-HF trial, and addresses the importance of a blind study design for further studies in this field. Moreover, this paper highlights future perspectives for examining the Algisyl implants, aimed at functional clinical outcomes, quality of life, and safety issues, prior to a possible implementation of this strategy into clinical practice.
INTRODUCTION
Remodelling of the heart is the term often used in the context of cardiac architectural changes (hypertrophy and dilatation) after myocardial infarction (MI), and in heart failure (HF).1 At present, pharmacotherapy, including angiotensin- converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, diuretics, and aldosterone antagonists, alongside revascularisation procedures, such as the percutaneous coronary intervention, with a stent implantation performed after MI, have given rise to a substantial reduction in left ventricular (LV) aneurysms, as well as an improvement in clinical outcomes.2,3 Nevertheless, in many post MI patients, a loss of contraction by the anterior wall and septum occurs, and the resulting LV dysfunction often leads to chronic HF.1,4 Over the past few decades, there have been various surgical attempts to reverse cardiac remodelling; however, some were unsuccessful.5 Therefore, to fulfil the unmet needs of a growing patient population with chronic post-MI HF, new, safe, and effective strategies are needed. One such intervention, based on a calcium alginate hydrogel, Algisyl2,3 (LoneStar Heart Inc., Irvine, California, USA), was examined in a recent AUGMENT-HF clinical study.6,7
UNIQUE CHARACTERISTICS OF ALGINATE-HYDROGEL BIOMATERIAL
Algisyl alginate, originating from brown seaweed, is a proprietary calcium-alginate-hydrogel that consists of two components: a sodium ion alginate component, supplied as a sterile aqueous solution with 4.6% mannitol, and a calcium ion-alginate component, consisting of water-insoluble particles suspended in a sterile 4.6% mannitol solution. These two components are mixed immediately before use, and then combined in one syringe for delivery as intramyocardial injections. When mixed, the Algisyl forms a solid hydrogel in the ventricle. This permanent implant, which is directly injected into the LV muscle, plays the role of a prosthetic scaffold that modifies the shape and size of the LV.6,7 It should be noted that alginate, which is known to be a safe biomaterial, has been used for many years in preclinical studies as a biologically inert filler substance.8 Moreover, it has been reported that the alginate hydrogel curtailed LV remodelling after MI in animal models.8,9
ALGISYL IMPLANTS: A MINIMALLY INVASIVE MYOPLASTY PROCEDURE
In the AUGMENT-HF trial, the Algisyl implants, which were inserted through a ministernotomy, negating the use of the much more invasive coronary artery bypass grafting surgery, were then injected directly into the heart muscle.6,7 At present, a limited left thoracotomy is required with a subsequent identification of the mid-wall of the beating heart. This is followed by a series of 0.3 cc Algisyl injections performed circumferentially at the LV mid-ventricular level with an average of 15 implants per ventricle, roughly 1 cm apart. This procedure takes about 80 minutes and, depending on the patient’s overall condition, the length of hospitalisation varies from approximately 3 days to a few weeks. It should be noted that some of these patients have to face a very difficult decision on whether to implant an LV assist device or whether to undergo heart transplantation. In this context, the potential therapeutic option of injecting Algisyl would be less invasive than the above two options. In the future, when the percutaneous device will be available to inject Algisyl endocardially, it is conceivable that this procedure could also be performed by invasive cardiologists.
AUGMENT-HF TRIAL: A BRIEF OVERVIEW
AUGMENT-HF10 (Table 1) is a randomised controlled trial, prospectively comparing Algisyl injections (intervention group) with the standard medical therapy (SMT) alone (control group) to determine the impact of Algisyl on functional capacity and clinical outcomes in patients with advanced, chronic HF.6,7 The primary endpoint of AUGMENT-HF included the change in peak maximal oxygen consumption (VO2) (from baseline to 6 months), measured during cardiopulmonary exercise testing. The secondary endpoints included changes in the 6-minute walk test (6MWT) distance and in the New York Heart Association (NYHA) functional class (Table 2).6,7 In addition, the assessments of safety (e.g., serious adverse events [SAE] and postoperative cardiovascular [CV] mortality) were conducted (Table 1).6,7 It has been shown that 35 patients in a modified intention-to-treat population from the intervention group were successfully treated with the alginate-hydrogel injections, administered through a limited left thoracotomy approach. These patients achieved an improved peak VO2 of 1.24 mL/kg/min from baseline to 6 months compared with the control group (Table 2).6 Similarly, after 6 months, patients in the intervention group improved by a mean of 84.7 m on the 6MWT compared with baseline; in contrast, the patients in the control group deteriorated, with an average of 15.4 m or less on the 6MWT, compared with baseline. The NYHA class was also reduced by one in the intervention group, while it remained the same in the control group.6,7
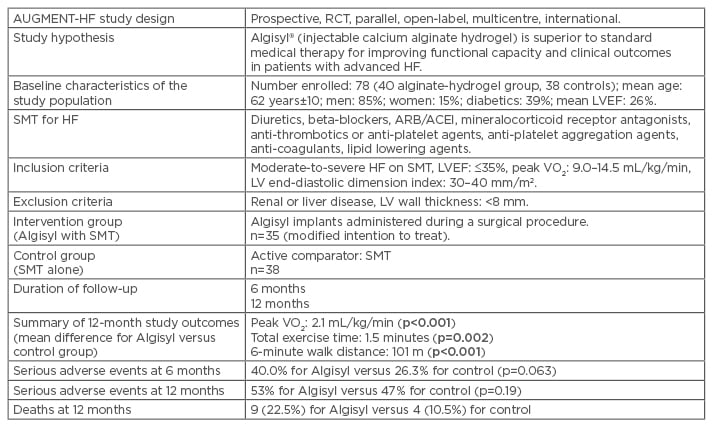
Table 1: Synopsis of the AUGMENT-HF trial.6,7
Data are presented as means±SD. p values in bold are considered statistically significant.
ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; HF: heart failure; LV: left ventricular; LVEF: left ventricular ejection fraction; RCT: randomised controlled trial; SD: standard deviation; SMT: standard medical therapy; VO2: maximal oxygen consumption.
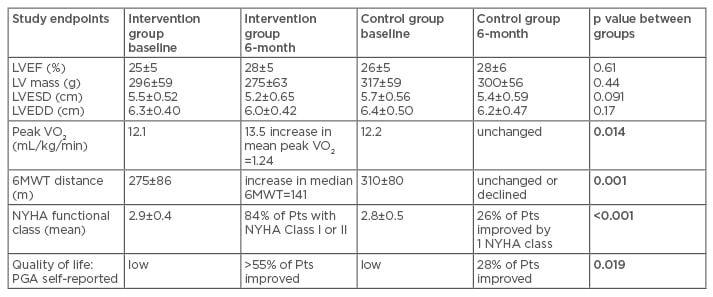
Table 2: Comparison of clinical outcomes between intervention group (Algisyl® injections) and control group (standard medical therapy) in the AUGMENT-HF trial.6
Data are presented as means±SD. p values in bold are considered statistically significant.
HF: heart failure; LV: left ventricular; LVEF: left ventricular ejection fraction; LVEDD: LV end-diastolic diameter; LVESD: LV end-systolic diameter; 6MWT: 6-minute walk test; NYHA: New York Heart Association; PGA: patient global assessment; Pts: patients; SD: standard deviation; VO2: maximal oxygen consumption.
Overall, the alginate-hydrogel was more effective than SMT alone for improving exercise capacity and decreasing HF symptoms.6,7 There was no significant difference in the number of SAE at 6 and 12 months between the intervention and control groups.6,7 The results of the AUGMENT-HF trial provided a proof of concept for possible LV reconstruction with Algisyl injections as a potential therapy for patients with advanced HF.6,7 However, the trial findings are far from conclusive; it is expected that future studies will explore the potential long-term benefits, as well as safety issues, for this innovative therapy.
CLINICAL IMPLICATIONS AND CHALLENGES RELEVANT TO RESEARCH ON ALGISYL
The recent report of the AUGMENT-HF study is the first step towards a more comprehensive treatment using injectable biomaterials for patients with advanced HF. It should be emphasised that the HF research focussed on early phase trials of novel interventions faces a big challenge, since such studies are usually underpowered to demonstrate the improvement in evidence-based clinical outcomes, including hospitalisation, SAE, and CV mortality.11 For this reason, such trials have to rely on other clinically relevant endpoints, including surrogate endpoints, for example, physiological parameters intended to predict outcome benefits, which are considered in larger trials.11 Since beneficial changes in the LV remodelling have been associated with improved clinical outcomes in larger studies, the AUGMENT-HF trial may offer some hope that, in the future, the intervention with Algisyl implants, aimed at the reshaping of failing LV, can really improve functional status of patients suffering from advanced HF.12
In the meantime, however, different issues, such as biological response to the Algisyl implant, beyond the mechanical benefits that could possibly result in alterations of myocardial structure or function, need to be explored.6,7 There is also concern surrounding an unblinded study population; for instance, the intervention group had a limited median sternotomy and surgical insertion of the Algisyl implant and as such these patients were aware of the therapeutic procedure. In contrast, the patients in the control group did not have this procedure. Under these non-blind circumstances, when the 6MWT was analysed, both the study patients and physicians knew who received an intervention. In this setting, it was possible that some expectations with regard to the outcome among patients in the intervention group might have some positive impact on the test results; the patients, who were aware of receiving the real intervention, could have performed slightly better on the 6MWT than the ones in the control group.6,7 However, the VO2 was an objective parameter of their functional capacity, which was measured by blinded adjudicators.6,7 In the future, randomised controlled trial designs need to address with caution the issue of blinding to avoid a potential bias.
FUTURE PERSPECTIVES: AUGMENT-HF II
As a follow-up of AUGMENT-HF, the AUGMENT-HF II trial has recently been approved by the U.S. Food and Drug Administration (FDA). This trial will enrol >250 patients and will use a similar design, including patient randomisation to the intervention group, which will receive the Algisyl implants versus the control group, which will receive SMT for HF. The study endpoints include peak VO2, hospitalisations due to HF, and CV mortality. In addition, the AUGMENT-HF II will focus on measurements of the quality of life.7
ALGISYL IN CONTEXT OF OTHER BIOMATERIALS CONSIDERED FOR MYOCARDIAL INJECTION POST MYOCARDIAL INFARCTION
Several approaches using specific biomaterials exist to help injured myocardium post MI. They include scaffolds (from natural or synthetic biomaterials), decellularised extracellular matrix (which can be applied to create cardiac patches), techniques to vascularise scaffolds, and injectable biomaterials (designed for endogenous or exogenous repair, or to maintain ventricular architecture after MI).12 In particular, the hydrogels class of biomaterials designed for direct injection into the heart can be divided into three main groups. Firstly, hydrogels for prevention of adverse remodelling and for recruitment of endogenous cells for repair; secondly, a temporary matrix for cell transplantation and exogenous repair; and thirdly, a support to the failing LV, by maintaining ventricular architecture after MI and promoting functional LV improvement.12,13
Currently, the injectable biomaterials are the focus of translational research, and they include:
- The first group, which contains naturally derived compounds, such as alginate, collagen, hyaluronic acid, fibrin, keratin, chitosan, and decellularised extracellular matrix.
- The second group, in which the main representatives include synthetic hydrogels; for example, self-assembling peptide or polymer-based materials that can augment stem cell survival or retention in vivo, enhance the release of growth factors, or even enhance endogenous tissue regeneration.12
Injectable hydrogels were initially investigated to augment cell retention and survival associated with cell transplantation in the heart. According to current knowledge, the injected cells act via paracrine mechanisms. Therefore, the main purpose of introducing a biomaterial scaffold is to augment their survival by providing the cells with an extracellular matrix that will enhance the beneficial paracrine signals.12 It has been shown that the cell retention was significantly improved after a direct epicardial injection of bone marrow-derived mononuclear cells in self-assembling peptide nanofibres in a large animal MI model (resembling a human MI model). The cell injection within the nanofibres contributed to better LV haemodynamic functions (both systolic and diastolic) as well as an increased capillary density.14
In recent years, some preclinical studies have explored the release of growth factors, therapeutic agents, and biologics from injectable scaffolds, in order to prevent negative LV remodelling. For instance, it has been found that the vascular endothelial growth factor release can be sustained for 2 weeks from self-assembling peptide nanofibers in vivo. Overall, in a large animal MI model, the nanofiber-vascular endothelial growth factor system improved angiogenesis and decreased infarct size after a direct epicardial delivery.15 Likewise, in another experimental MI model, it was shown that the myocardial extracellular matrix hydrogel, delivered via a transendocardial approach, improved the LV end-diastolic and systolic volumes, left ventricular ejection fraction, and global wall motion index. Overall, it has been shown that the hydrogel improved LV architecture and ability to contract.13 Although some preclinical studies appear promising, the exact therapeutic potential of biomaterials for HF has yet to be determined, based on large scale clinical trials. The translation of these therapeutic strategies into clinical practice faces several challenges that are mostly relevant to the manufacturing processes, methods of delivery, quality control, and complex regulatory and financial issues.12,16
Alginate biomaterial has been explored for potential applications in cardiac tissue engineering and regeneration due to its biocompatibility and non-thrombogenic nature.16 Biomedical applications of alginate range from its supportive use in patients after acute MI to its possible use as a vehicle for stem cell delivery, and for the controlled delivery and presentation of multiple bioactive molecules and regenerative factors into the heart.16 In conclusion, it should be noted that some properties of alginate, when taken separately, are not unique; however, the fact that the alginate combines all of these properties makes it a remarkable material that could be helpful to many patients suffering from severe myocardial damage after MI.
SUMMARY
Post-MI remodelling of the heart independently contributes to HF progression and, thus, there is a great interest in innovative approaches to attenuate and reverse LV remodelling. The AUGMENT-HF trial revealed that Algisyl, as a permanent implant designed to reduce the LV wall stress, is feasible, safe, and translatable into improvement of functional capacity. For example, improvements to peak VO2 and 6MWT have been sustained in 1-year follow-up in patients with MI and advanced HF. Further studies, including the AUGMENT-HF II trial, examining the Algisyl implants will focus on functional, clinical outcomes, quality of life, and safety issues such as SAE and CV mortality, among HF patients. Unquestionably, larger multicentre randomised, blinded studies are essential for evaluation and conclusion of clinical efficacy of this innovative therapeutic strategy.








