Abstract
Glomus tumours are benign neoplasms with classical presentations of cold intolerance, severe pain, and tenderness, primarily in subungual locations. However, these hallmark symptoms may not always manifest in extradigital sites, complicating the diagnostic process. This case report presents a patient with a 7-year history of debilitating pain due to a glomus tumour located in the right upper arm. Key clinical findings included persistent, unexplained pain without classic glomus symptoms. This case report highlights the importance of recognising atypical presentations of glomus tumours and the pivotal role of MRI in diagnosis. Optimal diagnostic outcomes are achieved when MRI is performed with a well-informed clinical background and close coordination between the patient, surgeon, and radiologist. Precise anatomical localisation, achieved through careful site marking, along with a reduced field of view in imaging, enhances lesion detection and further aids in minimising intraoperative challenges. This approach ensures more accurate diagnoses and improves surgical outcomes in cases where classical symptoms are absent.
Key Points
1. A brief introduction to atypical presentations of the glomus tumour and the importance of identifying them as classical symptoms may be absent, making its diagnosis clinically challenging.2. Explores strategies to enhance MRI outcomes, emphasising the importance of detailed clinical background and close collaboration between the patient, surgeon, and radiologist to achieve greater diagnostic precision.
3. Emphasises the significance of precise site marking, the correlation between field of view and resolution, and their roles in reducing intraoperative challenges while optimising surgical outcomes.
INTRODUCTION
Glomus tumours are uncommon, benign neoplasms originating from the glomus body, predominantly affecting adults between 20–40 years of age.1 Clinically, they often manifest as small, bluish, or reddish lesions, typically located in the subungual region of the distal extremities.1 Though glomus tumours are diagnosed clinically and confirmed through histopathological studies, due to the atypical and non-specific nature of their presentation, preoperative MRI imaging is instrumental in the diagnosis of extradigital glomus tumours.2,3 Surgical excision remains the gold standard for treatment, consistently yielding excellent functional and cosmetic outcomes and immediate pain relief.4 Following the Surgical CAse REport (SCARE) guidelines,5 the authors present a case involving a patient with a 7-year history of a painful lesion on his right upper arm. Despite undergoing 14 prior MRI scans, the definitive diagnosis was achieved with the 15th MRI, which ultimately led to the resolution of his pain.
PATIENT INFORMATION
The patient, a 42-year-old pharmacist of Indian ethnicity, presented with debilitating pain in his right upper arm, which had been present since 2017. He reported that the pain significantly restricted his daily activities and adversely affected his quality of life, including preventing him from lifting his young child. He was a non-smoker and had been using over-the-counter analgesics, in addition to multiple prescription pain medications, including gabapentin. Approximately 1 year prior, he was also prescribed antidepressants, which he took for 3–4 months. Before his presentation at our hospital, he sought medical help on three different continents, underwent 25 CT scans and 14 MRI scans, experienced a failed surgical attempt to excise the unidentified lesion, and was prescribed strong analgesics, all without significant relief. In 2024, the patient presented to the authors’ neurosurgery department, where a provisional diagnosis of neuroma was made. He was subsequently referred to the plastic surgery department.
CLINICAL FINDINGS AND DIAGNOSTIC ASSESSMENTS
The patient had no significant past medical history and no known allergies. The only notable symptoms were severe pain and tenderness at the antero-medial side of the right upper arm. On physical examination, he was alert, orientated, and vitally stable. Tenderness and a surgical scar from the previous procedure were noted in the right upper arm. Love’s test was elicited by pinpoint localisation of tenderness.6
Examination of the remaining organ systems showed no abnormalities. Laboratory investigations were within normal limits, including complete blood count, C-reactive protein, liver function test, renal function test, lipid profile, and blood glucose. An MRI with contrast and Doppler ultrasound were performed. The clinical scar site was localised, and a skin marker (pumpkin seed oil, in this case) was placed at imaging time. A well-defined, solid enhancing nodule in the subcutaneous plane in the right proximal arm was noted. The nodule was located at the site of the clinical skin scar, 3.2 mm deep to the skin surface. The nodular lesion measured 6.2×4.2 mm in size (Figure 1). It had smooth margins with signal intensity on T1-weighted sequence and intermediate signal on T2-weighted sequence. The postcontrast study demonstrated intense homogeneous enhancement in the lesion. The perilesional planes were smooth and well preserved. No deeper extension into the muscular plane and no prominent arterial feeder vessels were seen close to the mass.
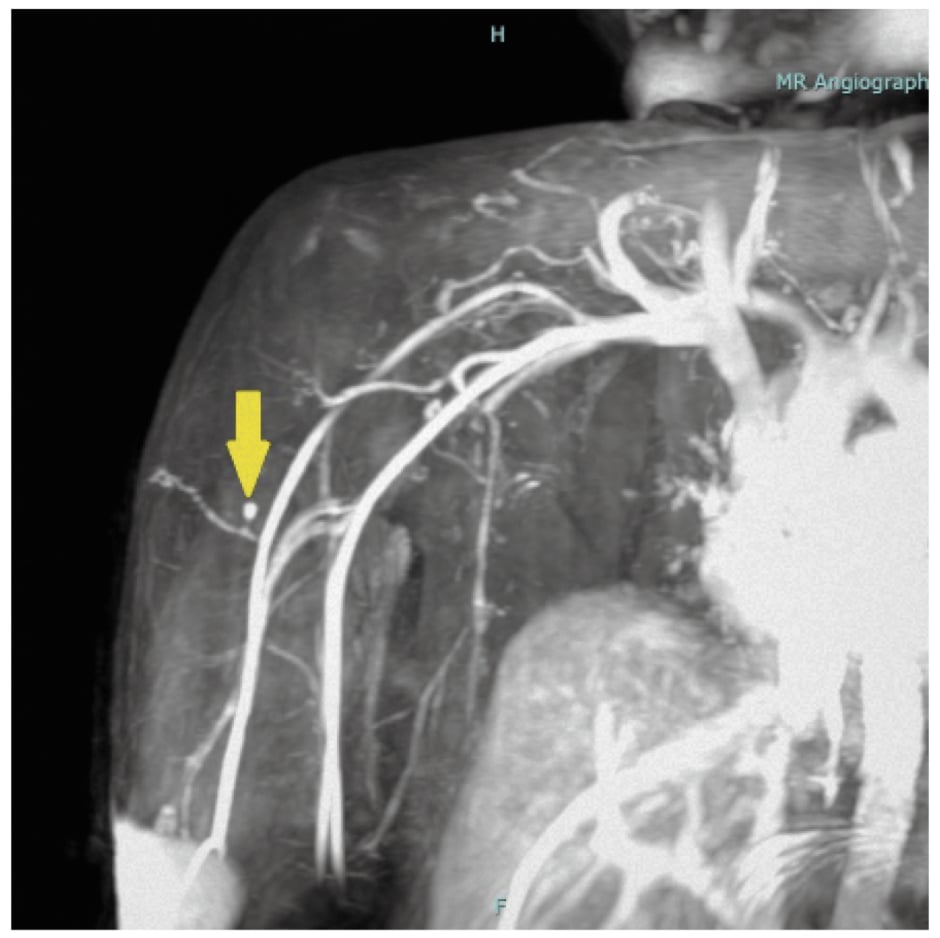
Figure 1: MR Angiograph.
MRI with contrast showing the nodular lesion measuring 6.2×4.2 mm in size and no prominent arterial vessels.
THERAPEUTIC INTERVENTION
Following the establishment of a working diagnosis and radiological localisation in reference to the previous scar and depth from the skin surface, microscopic excision of the tumour was promptly undertaken (Video 1). The microscopic excision was performed under 5x magnification with an incision made at the previous scar site, guided by the marker placed during MRI. The lesion was located and confirmed to match the MRI findings. Excision and haemostasis were achieved, followed by layered closure. The excised mass was non-encapsulated and measured approximately 6×4 mm (Figure 2). The absence of a tourniquet caused the surrounding fat to appear similar to the lesion, complicating identification (Video 2). It was, however, distinguished by its firmer consistency. Contrary to MRI findings, converging vessels were observed intraoperatively, giving the tumour a denser colour, visible under an operative microscope.
Video 1: Intraoperative glomus tumour measuring about 0.5 cm. Unlike the MRI finding, it was highly vascular.
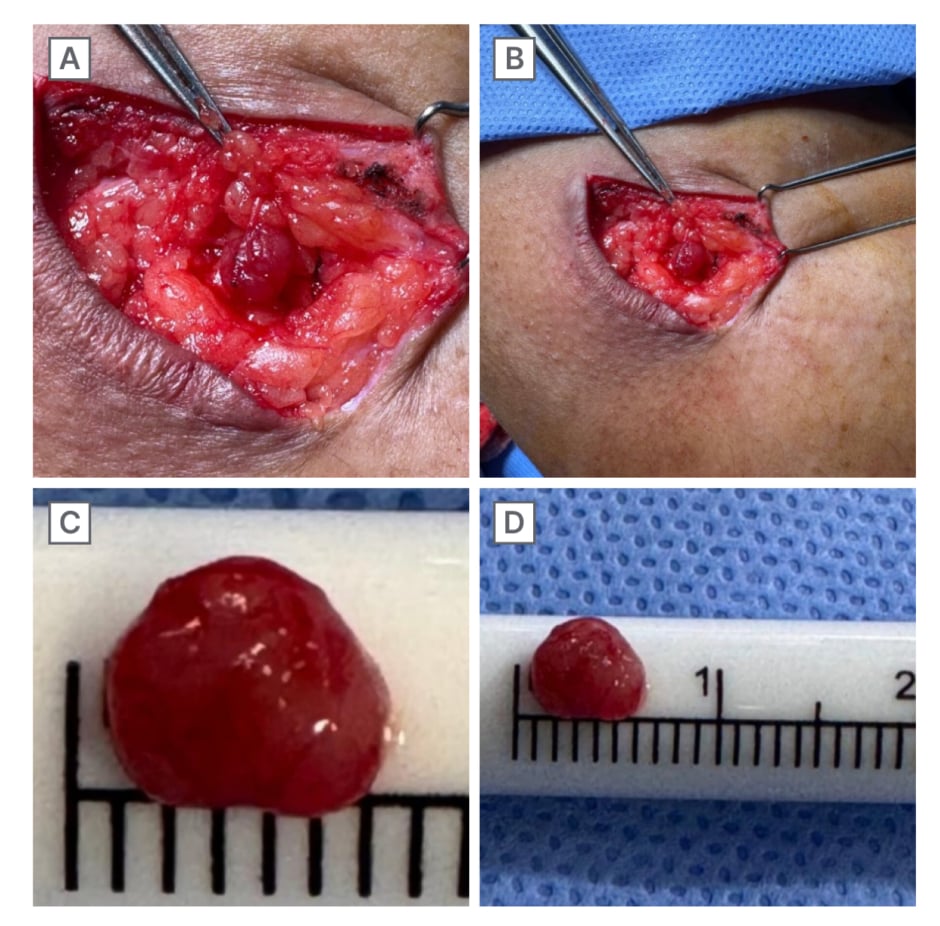
Figure 2: Intraoperative lesion, indistinguishable from the surrounding adipose cells with converging vascular
supply (A and B), and post-excision glomus tumour measuring 6 mm in diameter (C and D).
Video 2: Due to the absence of a tourniquet, the intraoperative glomus tumour was indistinguishable from the surrounding adipose tissue, identified due to the size marking, findings of the MRI, and vascularity.
The excised lesion was then sent for histopathological examination to confirm the diagnosis. Gross examination revealed a single, grey-brown irregular nodular mucosa-covered soft tissue, measuring 0.4×0.3×0.2 cm. Haematoxylin and eosin (H&E) staining demonstrated a well-circumscribed vascular tumour, characterised by capillary-sized vessels lined by endothelial cells, surrounded by glomus cells arranged in nests, sheets, and trabeculae within a myxoid stroma (Figure 3). The cells were polygonal, with moderate cytoplasm, indistinct borders, and rounded nuclei with amphophilic to eosinophilic cytoplasm. The chromatin appeared homogeneous and bland, with inconspicuous nucleoli and no detectable mitotic activity. These histopathological findings confirmed the diagnosis of a benign glomus tumour.
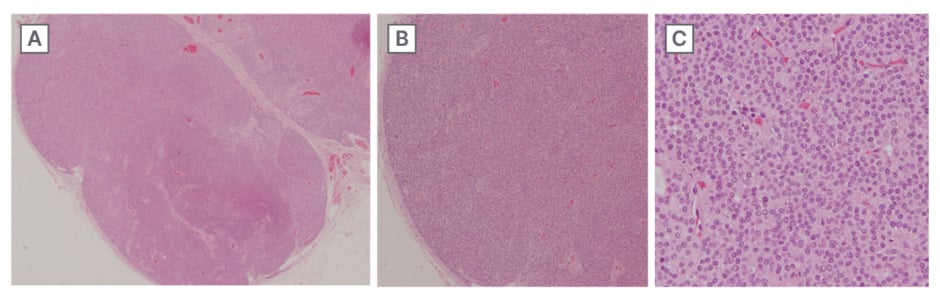
Figure 3: Hematoxylin and eosin staining.
A) Original magnification x40. Well-circumscribed vascular tumour. B) Original magnification x100. Capillary-sized vessels lined by endothelial cells surrounded by glomus cells forming nests, sheets, and trabeculae in a myxoid stroma. C) Original magnification x400. Nests and sheets of equally spaced polygonal cells with moderate cytoplasm. Cell borders are identifiable.
FOLLOW-UPS AND OUTCOME
Postoperatively, the patient experienced mild soreness at the incision site, which diminished over the next few days. Sutures were removed on the 8th postoperative day, and the patient was completely symptom-free by 2 weeks. He was followed up in the outpatient department for 6 months, during which his symptoms did not recur, and he was able to resume sports activities. A duplex ultrasound scan was performed to assess for any anomalous vascular patterns at the surgical site, confirming normal findings.
PATIENT PERSPECTIVE
Chronic pain can disrupt a patient’s social interactions, limiting their ability to engage with their social circles and family. Almost half of those with chronic pain have experienced reduced contact with their family due to their symptoms.7 This patient experienced intense pain for almost 7 years, where even the slightest touch would provoke extreme discomfort. In the quest to cease his pain, he relentlessly sought medical care, was put on strong analgesia, and underwent serial CT and MRI scans. The pain greatly restricted his daily activities, preventing him from even lifting his young child. The surgery resolved the pain completely and helped the patient return to a normal life.
DISCUSSION
Glomus tumours, first described in 1812, are benign neoplasms comprising approximately 1% of all hand tumours and around 2% of soft tissue tumours.8,9 Also referred to as glomangioma, glomangiomyoma, and glomangiomatosis, these tumours commonly arise in the distal phalanx, particularly in the subungual regions, due to the aberrant functioning of the glomus body.10 The glomus body, an arteriovenous anastomosis primarily located in the fingers and toes, is responsible for thermoregulation. The classic clinical presentation of glomus tumours includes a triad of cold intolerance, severe paroxysmal pain, and tenderness; however, these symptoms are less pronounced in extradigital cases.8 Extradigital glomus tumours can occur anywhere, including the wrist, forearm, elbow, shoulder, thigh, cheek, earlobe, foot, nose, and trachea.11 The diagnosis is primarily clinical and is confirmed through histopathological examination of the tumour. In the case of atypical presentations, the average duration of symptoms exceeds 7 years, with many patients having undergone prior evaluations where their conditions were misdiagnosed.11 However, MRI can help reach a diagnosis. The recurrence rate of glomus tumour is about 4–15% of cases with bone involvement on radiograph present in a few cases.12,13
The efficacy of diagnostic tests for glomus tumours can be significantly enhanced by implementing a targeted scanning protocol that is informed by a thorough clinical background. When clinical suspicion is high, targeted MRI protocols tailored to the suspected anatomical region can greatly improve the detection and characterisation of these lesions. Such protocols may include high-resolution imaging with specific sequences that highlight the vascular nature of glomus tumours and marking the clinical site of suspicion. Therefore, this protocol may facilitate a more accurate diagnosis. This approach underscores the importance of integrating detailed clinical information with advanced imaging techniques to optimise diagnostic accuracy and treatment outcomes.
In the context of MRI for diagnosing glomus tumours, the relationship between the field of view and resolution is a critical factor that influences diagnostic efficacy. The field of view refers to the extent of the anatomical area captured in the MRI image, while resolution refers to the level of detail that the image can reveal. As the field of view increases, the resolution typically decreases, and conversely, a smaller field of view allows for higher-resolution imaging. The authors selected a relatively smaller field of view in conjunction with a high-resolution MRI. The sensitivity and specificity of the scan were enhanced, therefore, leading to a diagnosis of a glomus tumour, which tormented the patient for almost a decade.
Additionally, the tumour’s location further challenged the surgical excision. Tourniquets are routinely employed to create a bloodless surgical field by temporarily restricting blood flow. This reduces intraoperative bleeding and allows for clearer visibility of anatomical structures and distinguishing highly vascular tissues from those with lower vascularity.14 Though a tourniquet could not be applied, the presence of a provisional diagnosis, anatomical reference from the previous scar, radiological localisation, and the presence of converging vessels that were not detected by the MRI ensured successful identification of the tumour intraoperatively.
Furthermore, close coordination between the patient, the treating surgeon, and the radiologist ensured that the diagnostic process was tailored to the patient’s clinical presentation and needs. This dialogue helped in confirming the diagnosis and formulating an effective treatment plan. When the patient is actively involved and informed throughout this process, it also enhances compliance with diagnostic and therapeutic recommendations, ultimately improving patient outcomes. Thus, close coordination among all parties not only improves the accuracy of the diagnostic tests but also ensures a patient-centred approach to care and the success of surgery.
CONCLUSION
Glomus tumours can present significant diagnostic challenges. While MRI is not inherently specific for glomus tumours, its diagnostic sensitivity can be markedly improved by using high-resolution imaging, precisely marking the site of interest, thereby reducing the field of view. Surgical resection of these tumours can be complex; however, incorporating a differential diagnosis and accurate anatomical site marking can facilitate the process. Effective communication between the patient, physician, and radiologist is crucial. This integrated approach improves diagnostic accuracy, especially in cases involving extradigital presentations.







