Abstract
Purpose: CT colonography (CTC) has been accepted as an optical colonoscopy (OC) alternative for colorectal cancer (CRC) screening by some guidelines, while others maintain that the data is insufficient. CTC’s less invasive nature may improve compliance; however, cost and need for colonoscopy, if lesions are detected, remain an obstacle for implementation. As a result, the authors set out to determine the cost-effectiveness of CTC in the context of its drawbacks and advantages when compared with OC within a Canadian context.
Methods: Using a decision analysis software, an economic analysis was performed comparing CTC to OC for CRC screening in asymptomatic patients. The 10-year primary outcome measure was study cost, cost difference of screening 100,000 patients, and the cost of one quality adjusted life year gained. The sensitivities, specificities, and polyp prevalence rates were derived from literature. The cost of each test was derived from local data.
Results: Local cost of OC is 764.36 CAD compared to 580.01 CAD for CTC. In the case of a normal OC, reassessment would not be necessary for 10 years, whereas in an asymptomatic average-risk population CTC must be repeated every 5 years. The incremental cost-effectiveness ratio, or the additional cost per life year of OC compared to CTC was calculated to be 3,390.76 CAD.
![]()
Erratum: CT Colonography Versus Optical Colonoscopy: Cost-Effectiveness in Colorectal Cancer Screening
Authors: Orysya Svystun, Marilyn Zeman, Michael Seidler, Christopher Fung
Original citation: EMJ Innov. 2022; DOI/10.33590/emjinnov/10035977 https://doi.org/10.33590/emjinnov/10035977.
Date correction published: 03.02.2023
The article by Svystun et al. in EMJ Innovations 7.1 was originally published on 24.10.22. Since then and erratum has been made. In Figure 1, “polypectomy” was misspelt as “polypectom” on several instances. This has now been rectified. In Table 2, the row “Incontinence pad” originally read “Incontinence underpaid” incorrectly. This has now been updated. In Table 3, the final row that reads “Total” had not been included. The table has now been updated to include this row.
EMJ apologises for the error and any inconvenience caused.
![]()
Key Points
1. In average-risk patients, colorectal cancer screening with optical colonoscopy is more cost-effective than CT colonography (CTC), as long as the CTC 5-year screening interval is maintained.2. In the context of a Canadian publicly-funded healthcare system, increasing the CTC screening interval to 10 years would render it a more cost-effective colorectal cancer screening modality compared with optical colonoscopy.
3. Patient compliance and a shorter time commitment in the absence of anaesthesia were not incorporated into the analysis, potentially underestimating the cost-effectiveness of CTC.
INTRODUCTION
In 2017, colorectal cancer (CRC) accounted for 13% of new malignancy diagnoses in Canada. It is the second leading cause of cancer-related deaths in Canadian males, and the third leading cause of cancer related deaths in Canadian females.1 Screening for CRC has been shown to reduce mortality, lead to earlier diagnosis, and consequently reduce costs for the healthcare system.2
The recently published US Preventative Services Task Force Recommendation Statement on screening for CRC offers numerous options for CRC screening, including yearly guaiac faecal occult blood test (gFOBT) or faecal immunohistochemical test (FIT); optical colonoscopy (OC) every 10 years; or CT colonography (CTC) or flexible sigmoidoscopy every 5 years.3 In this publication, they also advise initiating screening at 45 years (Grade B recommendation). The Canadian Task Force on Preventative Health Care (CTFPHC) suggests screening adults aged 50–74 years, who are not at high risk with either gFOBT or FIT every 2 years, or flexible sigmoidoscopy every 10 years.4 Positive screens are followed up with an OC.5 Meanwhile, the 2010 Canadian Association of Gastroenterologists (CAG) guideline recommends screening patients between 50–75 years, with the decision to screen individuals older than 76 years made on an individual basis. Screenings with gFOBT or FIT every 2 years, or with flexible sigmoidoscopy every 10 years are suggested. CAG recommends against CTC and OC as screening tools for the general population.6
Although Canadian participation rates in screening programmes have improved, they vary across the country, and in 2012 the target population had a rate of up to date CRC screening of 55.2%.7 The discomfort associated with OC and its complications likely contribute to the poor compliance with current screening recommendations.8 In addition, wait times for an OC after a positive gFOBT or FIT remain longer than targeted.9 Consequently, diagnosis most often occurs at Stage III CRC, which has negative implications on both the patient survival and treatment costs.4,10
CTC, also known as virtual colonoscopy, was developed as a less invasive alternative to conventional endoscopy. Patients undergoing CRC screening have been shown to prefer CTC to both OC and double contrast barium enema.11 While the technique requires colonic insufflation and bowel preparation, CTC eliminates risks of bleeding and colonic perforation, and has been shown to cause minimal adverse events related to radiation.8,12 Although CTC does not allow for polyp removal, its sensitivity for colonic lesions is comparable to endoscopy for lesions larger than 5 mm.8,13-16
Some analyses revealed that CTC, when contrasted with OC, may be less costly.17-19 Other reviews uphold that OC is more cost-effective, which continues to generate debate.20 Economic evaluation from one country may not be generalisable to another given variation in healthcare models, and unfortunately, the most recent Canadian-based cost-effectiveness analysis, which is limited by outdated sensitivity, specificity, and complication statistics, indicated that CTC was not cost-effective.21 To assess whether this still holds true in Canada, a single-payer healthcare system, the authors performed an economic evaluation comparing CTC to OC for CRC screening.
METHODS
An economic analysis, performed from the healthcare payer’s perspective, to compare CTC to OC for CRC screening was conducted using the decision tree software TreeAge (TreeAge Software Inc., Williamstown, Massachusetts, USA). The population of this hypothetical model was asymptomatic average-risk patients initiating screening at 50 years of age (corresponding to the current Canadian guidelines). The term polyp in this analysis refers to any polypoid lesion, including adenomas (serrated and sessile) and carcinomas that may be excised.
Cost of the two screening tests was calculated from local data within a publicly-funded tertiary care hospital in Canadian dollars. A 10-year period was elected for this analysis to account for the currently recommended OC interval.3,4 Subsequent 10-year cycles would have similar effects, and a longer period model was not analysed given screening duration variability depending on individual patients’ clinical context. CTC was compared to OC as OC was previously perceived as the more sensitive screening approach. The CTC and OC polyp sensitivity and specificity, and the incidence of procedure complications were derived from literature. The colonoscopy sensitivity averaged out to 83.7% for polyps larger than 6 mm, and 91.9% for polyps larger than 10 mm compared to 94.0% and 96.0%, respectively, in the last Canadian cost-effectiveness analysis. The colonoscopy specificity was 94.2% for polyps larger than 6 mm and 88.7% for those larger than 10 mm, whereas colonoscopy was deemed to be 100.0% specific in the 2005 publication. CTC statistics also differed with a sensitivity of 86.5% (61.0% in 2005) for lesions larger than 6 mm and 89.2% (71.0% in 2005) for polyps greater than 10 mm. CTC specificity is now 88.3% for polyps over 6 mm and 94.4% for those over 10 mm, compared to 84.0% utilised in the last Canadian analysis.21 Based on these values, a mathematical analysis was performed (Table 1). Of note, polyps smaller than 6 mm were not included in the analysis given the limited sensitivities and specificities documented in literature limiting their visualisation.8 To assess the value of the medical intervention in a more clinically relevant fashion, the authors utilised quality-adjusted life year (QALY). The value of 1 QALY equates to 1 year of good health. QALY values were derived from Jeong and Cairns.22 In congruence with the cost-effectiveness literature based on USA medical data, the authors set their QALY threshold at 50,000 CAD and 100,000 CAD.23,24 Institutional ethics research board approval was obtained prior to the initiation of this project (Pro00071780).
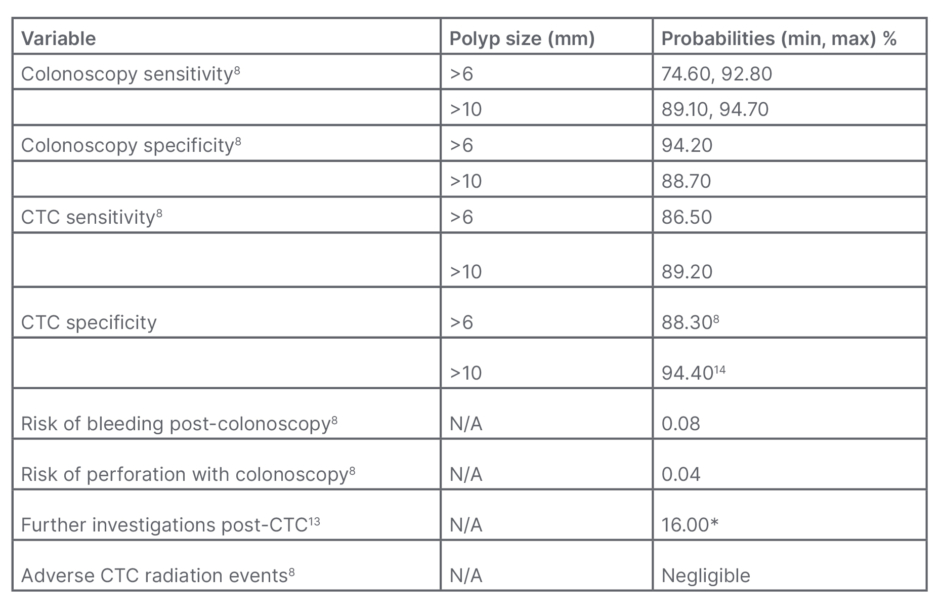
Table 1: Baseline probabilities.
*Extracolonic findings on CTC were found in 66% of the participants; however, only 16% required additional evaluation or urgent care.
CTC: CT colonography; max: maximum; min: minimum; N/A: not applicable.
As current guidelines recommend OC every 10 years and CTC every 5 years in average-risk individuals, patients would ideally receive two CTCs or one OC in the 10-year model period. The fact that patients would require an OC should CTC identify a lesion was accounted for in the analysis (Figure 1). Although CTC has the potential of improving screening compliance and has been shown to be preferred by patients over OC, this was omitted in the development of this cost analysis model and 100% adherence was assumed.25 Additionally, the indirect costs of CRC screening were excluded from the analysis; for example, the financial implications of the patient and individual driving them to the procedure being obligated to take time off from work, parking at the hospital, etc. The model also assumed endoscopy availability and patient compliance when a polyp was identified on CTC and required resection and colonoscopy. Consequently, polyp progression to cancer was not accounted for.
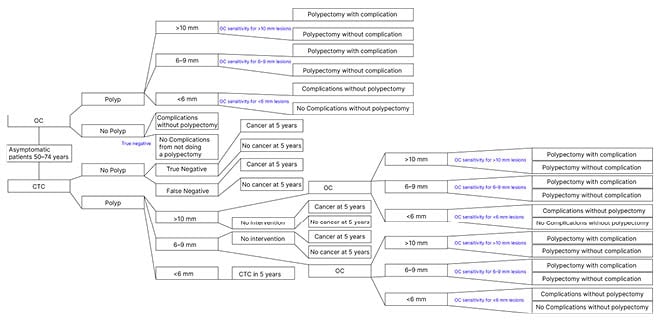
Figure 1: Cost analysis model.
CTC: CT colonography; OC: optical colonoscopy.
The study’s primary outcome measures consisted of the difference in cost between CTC and OC per individual study, per a 100,000-patient screening cohort, and finally the cost of 1 life year gained with each modality. Secondarily, the authors compared the CTC and OC cost per life year gained, and the financial burden of screening 100,000 patients if the CTC interval was increased to 10 years as opposed to the currently advised 5 years. This manuscript was prepared using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist for reporting economic evaluations of health interventions.26
RESULTS
A review of expenses at a local tertiary academic hospital revealed that the current cost per CTC study is 580.01 CAD while the cost for a diagnostic OC is 764.36 CAD, resulting in a difference of 184.35 CAD per investigation. When a polypectomy is performed, the fee for OC increases by at least 232.29 CAD. The aforementioned expense estimates account for nursing staff, physician billing, clerical work, equipment purchases and its maintenance, as well as linen services (Tables 2 and 3).
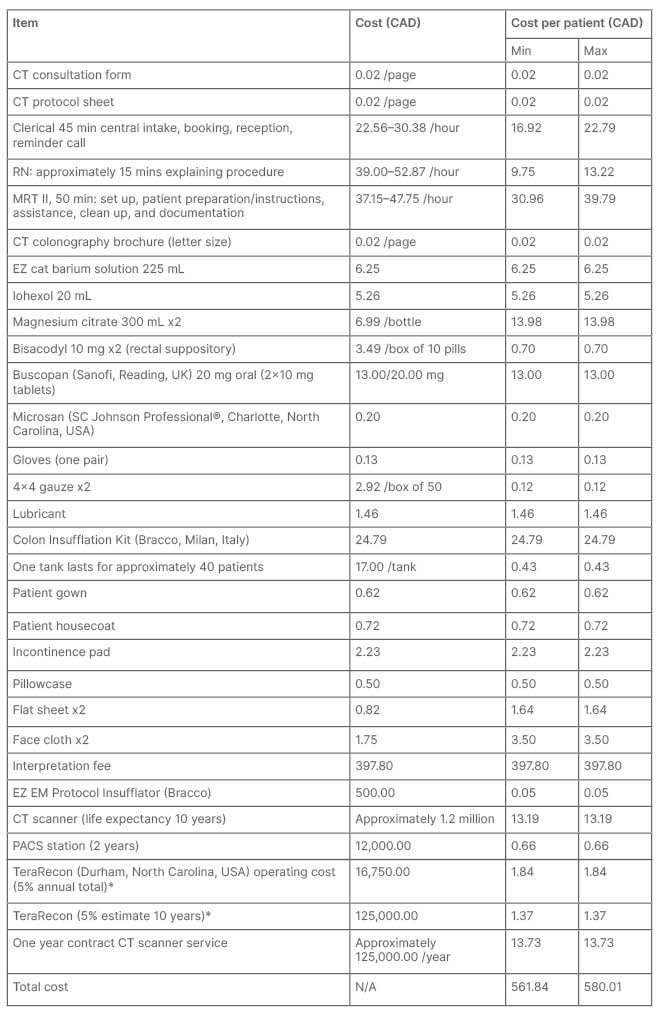
Table 2: Breakdown of CT colonography cost.
*The annual fee for the maintenance of the TeraRecon (Durham, North Carolina, USA) software is approximately 335,000 CAD. CTC is estimated to represent approximately 5% of TeraRecon’s local use.
Max: maximum; min: minimum; MRT: magnetic resonance tomography; PACS: picture archiving and communication system; RN: registered nurse.
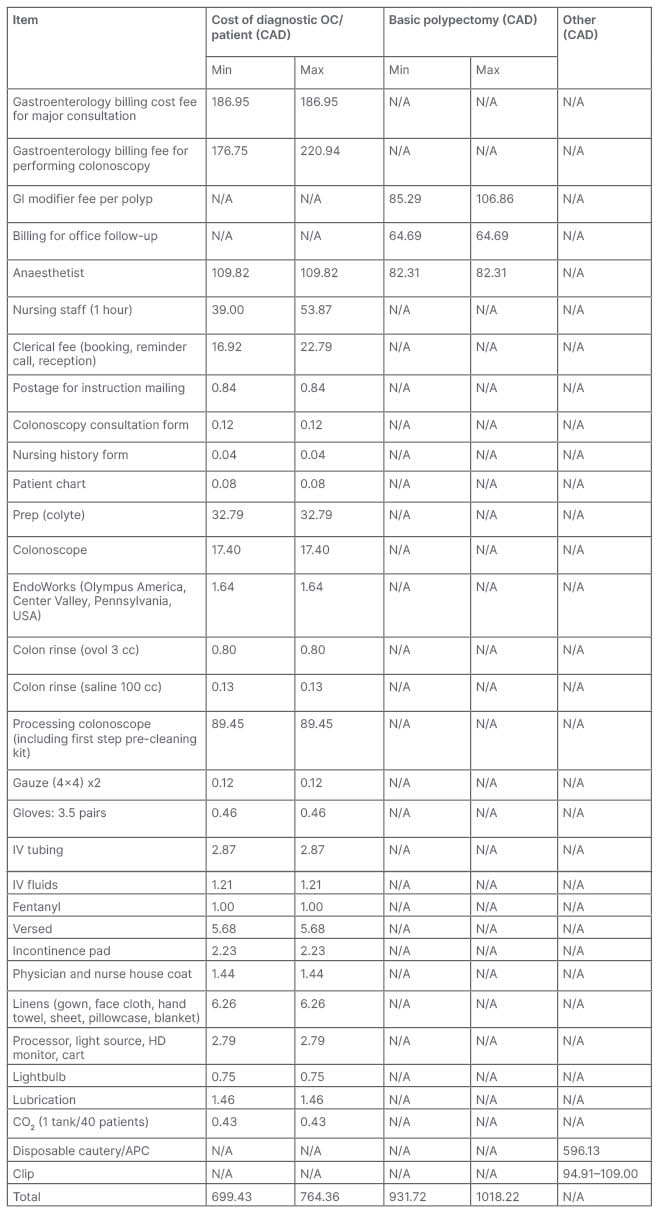
Table 3: Breakdown of optical colonoscopy cost.
APC: argon plasma coagulation; GI: gastrointestinal; HD: high definition; IV: intravenous; N/A: not applicable.
The 10-year cost prediction model demonstrates that OC remains the less expensive screening strategy. When 100,000 patients are screened with this method, 39.6 million CAD may be saved compared to screening patients with CTC. With QALY set to either 50,000 or 100,000, CTC becomes the more cost-effective method if the screening interval for CTC is increased to 10 years to mirror OC, rather than the currently recommended 5 years (Table 4).
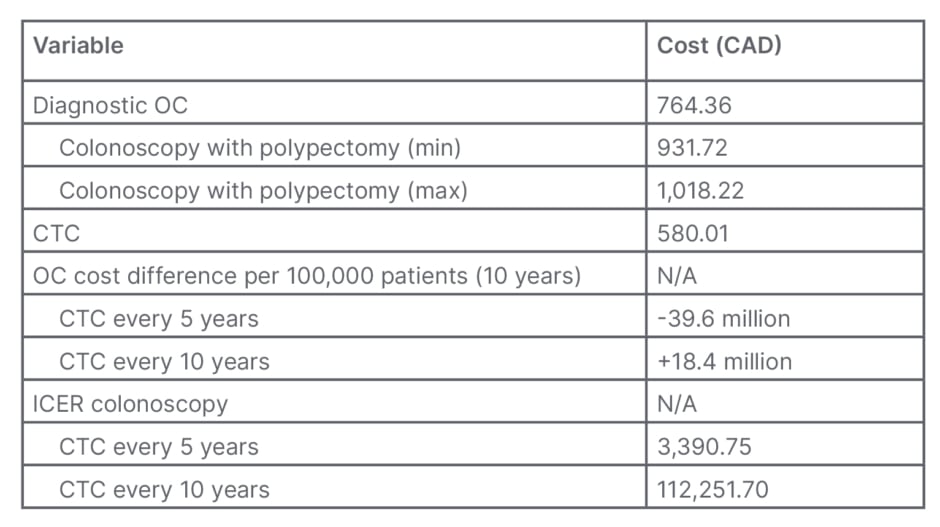
Table 4: Primary and secondary outcomes.
CTC: computerised tomographic colonography; ICER: Incremental cost-effectiveness ratio; max: maximum; min: minimum; N/A: not applicable; OC: optical colonoscopy.
DISCUSSION
CTC is a less invasive alternative to OC for CRC screening and has been suggested to be more economical. Although this study assumes 100% adherence and omits complication rates, it demonstrates that OC remains the more cost-effective screening tool if the CTC screening interval remains 5 years.
The finding that CTC is not cost-effective when applied to a 100,000-patient cohort was also the outcome of the most recent Canadian cost-effectiveness study comparing OC to CTC for CRC screening.21 Despite CTC costing 184.35 CAD less than OC per study and its sensitivity/specificity improving, these factors seem to be unable to overcome the costs associated with the need for OC when a tissue sample is needed. A study based on USA data also reproduced these results, assuming 100% adherence to screening and referral for OC in the presence of lesions greater than 6 mm; however, their cost differential per individual study was much smaller than that identified locally (488 CAD for CTC compared to 498 CAD for OC). Furthermore, Knudsen et al.27 created a model where CTC implementation was speculated to improve adherence to screening by 25%. With this assumption, despite CTC being only 10 CAD cheaper than OC, it became the most cost-effective method of monitoring for CRC even when compared to gFOBT.27 Interestingly, Pickhardt et al.28 submitted a response to this publication suggesting that a 25% increase in screening compliance could be easily met.28 Since then, a screening adherence improvement of 56% in the CTC arm has been shown in a randomised control screening trial.29 Finally, a study published in 2017, which compares cost-effectiveness of 13 CRC screening strategies, also showed that OC remains most cost-efficient. Of note, CTC every 10 years is the second most cost-effective, followed by sigmoidoscopy every 5 years.30
There are also a number of non-Canadian publications identifying CTC to be more cost-effective when compared to OC, as demonstrated by Pyenson et al.18 using data from 2013–2015 Medicare claims. In their analysis, CTC was valued at 25% of the cost of a diagnostic OC compared to 76% locally.18 Additionally, Sawhney et al.31 used retrospective healthcare claims from 2016 to show the average cost of OC was 2,033 CAD per insured patient. The authors state that insufficient CTCs were conducted to derive reliable values, and as a result CTC costs were estimated. Despite this bias, their analysis deemed CTC at least 22% less costly than OC, which was attributed to not needing anaesthesia or pathology services.31 Additionally, imaging for CRC screening was found to be cost-effective in a Dutch analysis comparing the two modalities (CTC was done every 5 years for a total of 25 years within their model and measured against OC, which was performed thrice over 30 years). Unlike this model, their publication used screening participation rates derived from literature, and accounted for complication costs by generating estimates for CTC.19 Finally, a randomised control trial based in Amsterdam and Rotterdam, the Netherlands, showed that CTC was more cost-effective than OC if CTC is used to screen more than twice in a lifetime, primarily due to improved participation rates.29 Unfortunately, as with the aforementioned articles, it is difficult to cross-reference expenses to local data but in van der Meulen et al.’s29 publication, a diagnostic CTC was 77% the cost of a diagnostic OC, which is similar to our local values.
An important point for discussion is the screening interval for CTC. Secondary to technological advances, CTC sensitivity and specificity is now near-equivalent to that of OC colonoscopy (Table 1). Consequently, an argument can be made for increasing the period between CTCs. The model where CTC and OC are performed with the same frequency shows that OC has an incremental cost-effectiveness ratio of 112,251.70 CAD, clearly falling outside of the historically utilised QALY of 50,000.00 CAD; however, Neumann et al.24 argue that the 50,000.00 CAD threshold is long outdated, and urge that 100,000.00 CAD serve as a new cut-off. Adopting this recommendation would not alter the fact that OC is not cost-effective should the CTC screening interval be increased to 10 years.
One of the potential drawbacks of this analysis is the omission of downstream complication costs of each modality. The 2016 US Preventative Services Task Force publication on CRC indicates that the risks of bleeding and perforation with OC are 0.08% and 0.04%, respectively.8 The same article also concluded that radiation associated complications of CTC are negligible; a statement that is further supported by many radiation scientists. There is no conclusive evidence that medical imaging radiation causes harm to adults especially given that the currently available techniques result in an effective dose equivalent or less than the annual background dose of 3 mSv.32 Johnson et al.13 found that CTC results in 16% of patients having further investigations. Interestingly, a retrospective cohort review of patients who underwent CTC revealed that within the year post screening they did not differ in their healthcare expenses compared to patients who had undergone OC.33 This suggests that omitting modality related complication costs is unlikely to have an impact on the cost-effectiveness analysis.
Johnson et al.13 also reported that 66% of patients undergoing CTC have extracolonic findings.13 Pickhardt et al.,34 back in 2009, showed that CTC is cost-effective secondarily due to its ability to identify abdominal aortic aneurysms and CRC simultaneously at no extra cost.34 Therefore, not accounting for extracolonic findings and their impact on cost-effectiveness may have devalued CTC. The cost-effectiveness of CTC may also be underestimated by this study by omitting from the analysis the income lost by the patient and whoever drives them to the procedure since following CTC. In the absence of sedation, the patient is safe to operate a vehicle, which is not the case with OC. Another drawback of this study is the omission of compliance rates to each modality for CRC screening. Although these statistics have been documented, no values were identified in Canadian literature. Consequently, an assumption of 100% adherence to both screening tests was adopted in this hypothetical model. As a result, variations in adherence were not considered as in the 2005 Canadian analysis.
CONCLUSION
There is continuous debate in literature regarding the cost-effectiveness of CTC in comparison to OC for CRC screening. This study is the first recent Canadian analysis on the subject and its results may be generalisable to other single-payer healthcare systems. It demonstrates that when a 10-year interval is considered for the monitoring of 100,000 people, OC remains the more economical approach, despite being the more costly investigation. Future directions could involve performing a similar analysis with the incorporation of published screening adherence statistics, as well as developing a model spanning the duration of recommended surveillance rather than the 10 years the authors assessed. Another potential area of research within a Canadian context is conducting a cost-effectiveness analysis of magnetic resonance colonography, which has only been previously done outside North America.19








