Meeting Summary
Cholestatic pruritus, or itch, is a prevalent and often debilitating symptom experienced by up to 89% of people living with primary biliary cholangitis (PBC). It can cause sleep problems, fatigue, social isolation, and, in extreme cases, suicidal ideation. Yet it is poorly understood, managed, and treated, making it a significant unmet medical need. A range of posters presented at the American Association for the Study of Liver Diseases (AASLD) ‘Liver Meeting 2024’ in November aimed to shine a light on the burden of pruritus and the importance of its identification, and to put forward validated measurement and monitoring instruments with the potential to enhance care.
The presented data showed that pruritus significantly impacts quality of life (QoL), increases healthcare resource costs, and decreases work productivity; but despite such profound effects, many patients report inadequate relief from existing therapies. The posters also indicate that validated patient-reported outcome measures (PRO) can accurately assess itch severity and its impact on QoL, with tools such as the PBC-40 and Worst Itch Numerical Rating Scale (WI-NRS) demonstrating consistency and reliability to clinical trial standards.
Understanding its severity and impact is crucial for effective pruritus management, and the key to improving patient care lies in its routine assessment. While the specific method of assessment may vary, the consistent use of PRO tools is essential in clinical practice to identify patients in need of intervention and to enhance their overall well-being.
Introduction
PBC is a progressive, chronic autoimmune liver disease. It causes a cycle of immune-mediated biliary epithelial cell injury, cholestasis, and progressive fibrosis that can culminate in end-stage biliary cirrhosis.1 It predominantly affects women over the age of 40 years, with an estimated prevalence of 39.2 per 100,000 people in the USA in 2014.2 Cholestatic pruritus is among the most common symptoms, affecting up to 89% of patients.3,4 In around 35%, the itch is persistent.5 Pruritus can have a significant impact on quality of life.4 Around three-quarters of patients with PBC who experience pruritus report disturbed sleep,6 which has been associated with fatigue and social isolation.7,8 In extreme cases, severe itch can even lead to suicidal ideations or be an indication for liver transplantation.8,9 Despite its huge impact, PBC-related pruritus is poorly managed and ineffectively treated.5
To date, four drugs have been approved for PBC disease management in the USA. Ursodeoxycholic acid (UDCA) is recommended as a first-line therapy, and obeticholic acid (OCA) as second-line.10 Elafibranor and seladelpar were both approved in 2024 for adjunctive use with UDCA in adults who have an inadequate response to UDCA, or as a monotherapy in people who are intolerant to UDCA.10,11 A number of potential therapies for cholestatic pruritus, including cholestyramine, rifampicin, naltrexone, and sertraline, have been found to be partially effective for pruritus in PBC, but none are approved by the FDA to treat pruritus in this specific population.4,8
Data shared at the AASLD 2024 annual meeting sought to increase understanding of the burden of cholestatic pruritus in PBC. A range of posters investigated the impact of cholestatic pruritus on patients’ health-related QoL (HRQoL) and on society at large, highlighted the need for more effective treatments, and presented validated PRO instruments that could help clinicians to consistently and reliably assess and manage this burdensome symptom.
Individual and Societal Impact of Pruritus
The poster presentations demonstrated that pruritus has a significant impact on a person’s life in a variety of ways, from their sleep patterns to their work productivity.
A retrospective cross-sectional analysis of adults living with PBC in the USA, for example, found that those with more severe pruritus and more frequent pruritus-related sleep disturbance had worse HRQoL across all domains of the PBC-40 instrument.12 The PBC-40 is a disease-specific HRQoL measure that evaluates patients’ experience of PBC across the six domains of symptoms, itch, fatigue, cognitive, social, and emotional. A total of 232 patients, all of whom enrolled in the PicnicHealth PBC Registry between January 2021–May 2022, completed at least one PBC-40 questionnaire. Overall, only 22 (9.5%) had no pruritus (PBC-40 itch domain score=0). Of the remaining 210, 88 (37.9%) had non-clinically significant (CS) pruritus, defined as a PBC-40 itch domain score of between 1–6, and 122 (52.6%) had CS pruritus, or a PBC-40 score of ≥7.12
The study found that the HRQoL impact escalated in line with itch severity (as measured by the PBC-40 itch domain scores), those with at least some pruritus exhibited significantly worse PBC-40 scores across all domains than those with no pruritus, and the same was true when comparing those with CS and non-CS pruritus. In addition, those with CS pruritus had statistically significantly higher odds of having CS PBC-40 scores in all domains (all p<0.0001) than those with non-CS pruritus. They also had higher odds of having worse emotional scores (odds ratio: 2.67; p=0.0006). The study also found that PBC-40 scores across all measured domains worsened significantly with increasing frequency of pruritus-related sleep disturbance (Table 1).12
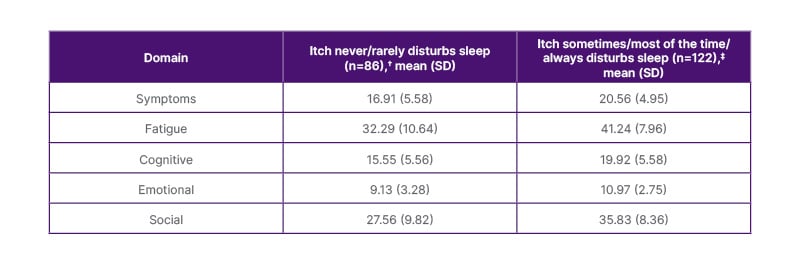
Table 1: PBC-40 domain scores worsened significantly* with increasing frequency of pruritus-related sleep disturbance.12
*p<0.0001 for all PBC-40 domain scores in patients where itch never/rarely disturbs sleep versus patients where itch sometimes/most of the time/always disturbs sleep. Thresholds for clinical significance (and the minimum and maximum possible score) for each of the domains were as follows: symptoms: 18 (6–35); fatigue: 33 (11–55); cognitive: 18 (6–30); emotional: 12 (3–15); and social: 32 (8–50).
†Emotional and Social, n=85.
‡Emotional and Social, n=121.
PBC-40: primary biliary cirrhosis 40; SD: standard deviation.
Similar findings highlighting the widespread impact of moderate-to-severe cholestatic pruritus on QoL were reported by the ITCH-E study.13 This real-world, cross-sectional, mixed-methods study, results of which were presented in another poster, assessed the impact of pruritus on HRQoL, as well as activity, and work productivity. Ninety adults with PBC were enrolled. Of these, 40 experienced no or mild pruritus (NMP), defined as a Pruritus Numerical Rating Score (NRS) of <4, and 50 experienced moderate or severe pruritus (MSP), defined as an NRS of ≥4. Participants completed questionnaires on six validated patient-reported outcome measures (PBC-40, Functional Assessment of Chronic Illness Therapy–Fatigue Scale [FACIT-Fatigue], Chronic Liver Disease Questionnaire-PBC [CLDQ-PBC], 5-Dimension Itch Scale [5-D Itch], European Quality of Life 5 Dimensions 5 Level [EQ-5D-5L], and Work Productivity and Activity Impairment Questionnaire [WPAI]).13
Compared with the NMP group, the MSP group had worse mean PBC-40 scores in the domains of symptoms (17.9 versus 20.9), itch (5.6 versus 8.9), fatigue (30.2 versus 37.4), cognitive (14.8 versus 19.1), and social (29.3 versus 35.9). They also had worse FACIT-Fatigue (32.2 versus 23.9), 5-D Itch (9.7 versus 14.4), CLDQ-PBC total (4.9 versus 3.9), and EQ-5D Index (0.78 versus 0.63) scores than the NMP group. In terms of productivity, the MSP group reported statistically significantly greater WPAI activity impairment than the NMP group (36.3% versus 56.6%; p<0.001). The MSP group also reported statistically lower work status compared with the NMP group (42% versus 53%). Among employed participants, the MSP group reported a greater probability of impairment while working and overall work impairment due to PBC; however, these differences were not statistically significant. The researchers postulated this could be related to the fact that fewer than half of participants were employed.13
A subset of the MSP group also answered open-ended questions regarding the impact of the disease and itching on their daily lives via voice response. The researchers identified a range of themes in the contributions, including unrelenting itching, with most participants describing their pruritus as “consistently intense”, and social and emotional impacts, with patients saying the pruritus caused them to self-isolate, avoid social activities, or stay at home. Around half said they felt judged while scratching, and several said they were embarrassed by it. A handful of participants said the itch distracted them from their work and made it “hard to focus”. Night-time itch may compound this by disrupting sleep and causing tiredness, suggested the authors. In severe instances, the itch can even force people to leave work or use paid time off to stay at home, they added.13
The impact of symptoms such as itch, however, is not confined to the individual patient, as demonstrated by a real-world, retrospective USA population-based study, also presented as a poster at AASLD, that found that patients with PBC who experience pruritus or fatigue have much higher healthcare resource utilisation and costs than those who do not.14 The study, which utilised the IQVIA PharMetrics® Plus database (Durham, North Carolina, USA), matched 760 adult PBC patients with pruritus to the same number of patients with PBC without the symptom. Likewise, 1,839 patients with PBC who experienced fatigue were matched to the same number of patients with PBC without fatigue. All were diagnosed between 2016–2022, and had ≥1-year continuous enrolment before and after the index date (defined as a random date with pruritus or fatigue recorded after the PBC diagnosis date for cases, and a medical visit date after the PBC diagnosis date for controls). All-cause per-patient per-year healthcare resource utilisation and healthcare costs during the 12 months following the index date were summarised and compared across cohorts. Healthcare costs were measured as total payments from payers, adjusted for inflation, and reported in 2023 in USD.14
During the 12-month follow-up period, the proportion of patients with emergency room (ER)- and inpatient (IP)-related healthcare resource utilisation was significantly higher in the pruritus and fatigue case cohorts compared with their matched controls. Among those with pruritus, 42.4% accessed an ER and 20.8% had an IP admission, compared to 28.0% and 6.1% of controls, respectively (p<0.001 for both). Among those with fatigue, 46.7% accessed an ER and 22.8% had an IP admission, compared to 27.8% and 7.2% of controls, respectively (p<0.001 for both). Patients within the pruritus and fatigue case cohorts had a higher number of IP admissions (pruritus: 0.44 versus 0.07; fatigue: 0.51 versus 0.09; p<0.001 for both), and longer length of IP stay (pruritus: 3.47 days versus 0.43 days; fatigue: 4.88 versus 0.65; p<0.001 for both), as well as higher number of outpatient visits (pruritus: 26.62 versus 16.32; fatigue: 29.90 versus 16.72; p<0.001 for both) and ER visits (pruritus: 0.99 versus 0.47; fatigue: 1.21 versus 0.45; p<0.001 for both) than those in the control groups. For most healthcare resource types, patients in the pruritus and fatigue case cohorts had significantly higher costs than their matched controls during the follow-up period (Figure 1).14
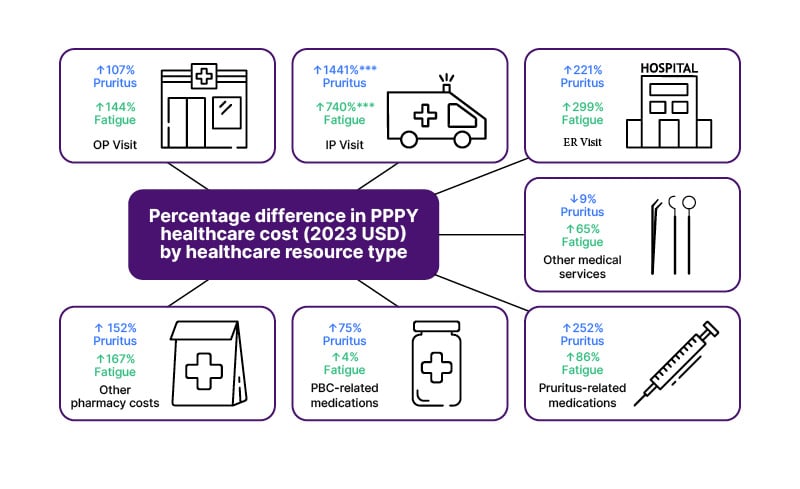
Figure 1: Percentage difference of annual healthcare costs of cases compared with matched controls across the 12-month follow-up period.14
A) Other medical services included durable medical equipment and dental or visual care; B) PBC-related medications included UDCA, OCA, fenofibrate, and gemfibrozil (all oral medications); C) Pruritus-related medications included cholestyramine, colestipol, colesevelam, rifampicin, naltrexone, topical calcineurin inhibitors, and anti-histamines, all of which are administered orally or topically; additionally, anti-histamines, while typically oral or topical, can also be administered via injection; D) Fatigue-related medications included rituximab, armodafinil, modafinil, fluoxetine, antioxidants, ondansetron, methotrexate, corticosteroids, and fluvoxamine; among them, corticosteroids, methotrexate, ondansetron, and rituximab can be administrated orally or via injection, and the remaining are all oral medications.
***Statistically significant (p<0.001) mean cost difference between the case and control groups.
ER: emergency room; OCA: obeticholic acid; OP: outpatient; PBC: primary biliary cirrhosis; PPPY: per-patient per-year; UDCA: ursodeoxycholic acid; SD: standard deviation.
Overall, total healthcare costs in the follow-up period were significantly higher for the pruritus and fatigue case cohorts than their matched controls. In the pruritus group, the mean cost across the 12-month follow-up period was 56,822 USD (standard deviation [SD]: 121,129 USD) among cases and 16,286 USD (SD: 27,859 USD) among controls, equating to a mean cost difference of 40,546 USD (95% CI: 33,860–47,131 USD; p<0.001). In the fatigue group, the figures were 61,167 USD (SD: 192,389 USD) among cases, and 18,651 USD (SD: 44,874 USD) among controls, with a mean cost difference of 42,515 USD (95% CI: 36,074–48,931 USD; p<0.001).14
Unmet Needs
The authors of all three studies concluded that their results highlighted the significant impact of itch, as well as the need for effective management.12-14 Yet it is currently poorly managed and ineffectively treated. A Phase IIb study of an investigational drug for PBC-related pruritus, for example, found that more than one third of the 147 participants experienced moderate-to-severe itch at baseline despite receiving concomitant itch medications.15,16 Building on this insight, investigators analysed data on 238 participants in a Phase III study of the same agent, 42% of whom experienced moderate (WI-NRS: ≥4–7) and 58% severe (WI-NRS: ≥7) pruritus at baseline.16 The results, presented as a poster at AASLD, found that while bile acid-binding resins are recommended as first-line therapy for itch in the USA and Europe,6,8 their use at baseline was low, at just 9% of participants. Furthermore, despite stable use of background itch therapies being permitted in the study, less than half (48%) were receiving any concomitant therapy for the symptom at all, while almost one-quarter (22%) were receiving fibrates for PBC and/or itch, yet were still experiencing moderate-to-severe pruritus at the start of the trial.16
Researchers also explored participants’ previous experiences with pruritus medication and found that the main reason for withdrawing therapy was a lack of efficacy. Antihistamines, for example, were the most common prior itch medication, taken by 15.5% of the cohort. However, in 81% of cases they were discontinued before the study started due to a lack of efficacy. Thirteen percent of participants had previous experience with bile acid-binding resins (cholestyramine and colesevelam), but the therapy was discontinued due to a lack of efficacy in 52% of cases, and due to a lack of tolerability in 32%. The only outlier was fibrates, which 3.4% of participants had received prior to starting the study, and where the main reason for discontinuation was treatment-emergent adverse events (75%).16
Studies to Validate Patient-Reported Outcome Instruments
Despite the prevalence and impact of pruritus in PBC, it is under-recorded in patient records, and its severity and HRQoL impact are not routinely assessed in clinical practice.17,18 Physicians use tools inconsistently, and different instruments have been used across different clinical trials, making comparisons challenging.18,19 Recent work in this area, however, could help enhance pruritus assessment, both in research and the clinic. Posters presented at AASLD demonstrated that PROs such as the PBC-40 and WI-NRS can be used to consistently and reliably assess quality of life and itch, to clinical trial standards.18,19
In one poster, researchers performed a psychometric evaluation of the tools used in the Phase IIb study mentioned earlier: WI-NRS, the Sleep Interference NRS, the Fatigue NRS, and a 7-day recall version of the PBC-40 (previously validated with a 4-week recall). Additional PROs, including the 5-D Itch scale, patient global impression of severity (PGI-S), patient global impression of change (PGI-C), EQ-5D, and Beck Depression Inventory-Second Edition (BDI-II), were used to support these psychometric evaluations. A range of measurement properties (including validity, reliability, responsiveness/sensitivity) were assessed for the NRS items and PBC-40 (7-day recall) in the intent-to-treat population (n=147) from the Phase IIb study.18
Analysis confirmed convergent validity for the NRS items and the PBC-40 (7-day recall) domains of Itch, Fatigue, Cognitive, and Emotional, based on moderate-to-large correlations with scores of similar concepts from other measures (all r≥0.46; p<0.0001) at baseline. The Sleep Interference NRS and Fatigue NRS discriminated between levels of severity in the expected direction across relevant measures (all p<0.0001). Domain scores of PBC-40 distinguished between patients grouped by various parameters, including EQ-5D scores (all p<0.01). In addition, results demonstrated that NRS and PBC-40 scores were highly consistent between the initial and retest periods. Responsiveness (sensitivity to change) of the WI-NRS (F value: 19.93; p<0.0001), sleep interference NRS (F value: 16.68; p<0.0001), and PBC-40 Itch domain score (F value: 16.26; p<0.0001) was supported when examining change scores against responses on the PGI-C. Sensitivity of the Fatigue NRS was also supported when examining change scores against PBC-40 Fatigue domain scores (F value of 8.97; p<0.0001).18
While the analysis described in this poster supports the use of these tools as valid, patient-centric clinical trial endpoints, the authors also suggest that their use in the routine clinical assessment of symptoms and HRQoL may improve care.18 This approach is further advanced by the results of another poster, which explored the correlation between pruritus severity as measured by WI-NRS and PBC-40 Itch domain, and concluded that either could be used to identify patients who could benefit from intervention.19 The poster pooled data from the same Phase IIb trial of 147 participants and an observational PRO validation study, which collected PRO data on 141 people over an 8-day period. In both studies, pruritus severity was assessed twice daily using the 0–10 WI-NRS. Worst daily itch (WDI) score was the higher of the two daily responses, and weekly itch score (WIS) was the average of the WDI scores over 7 days. HRQoL was assessed using the 7-day recall version of the PBC-40 and the EQ-5D. The Phase IIb trial also used BDI-II to assess depression symptoms.19
In the study, the investigators found that PBC-40 Itch domain scores increased in line with WI-NRS scores; there was a strong correlation (rs:0.69; p<0.0001) between PBC-40 Itch domain scores and WI-NRS, defined by WIS as measured by EQ-5D utility scores, which worsened with increasing WI-NRS scores. A WI-NRS of 10 corresponded with a mean EQ-5D utility score of 0.45 (SD: 0.34), indicating extremely poor HRQoL. The analysis also found moderate-to-strong correlations between EQ-5D utility scores and all PBC-40 domains (range: 0.41–0.56; p<0.0001) and a strong correlation between EQ-5D utility scores and BDI-II scores (0.61; p<0.0001). Mean BDI-II scores were higher (13.8–23.2) in those with severe pruritus (WI-NRS: ≥7).19
The poster further demonstrated that the severity cutoff used to define the moderate-to-severe population on WI-NRS (≥4) identifies the same population with clinically significant itch on the PBC-40 Itch domain (≥7). Severe pruritus on WI-NRS (≥7) corresponded to PBC-40 Itch domain scores of ≥10 (Figure 2).18 The authors note this is a slightly lower threshold for severe itch than previously proposed for the PBC-40 Itch domain (≥12).5,19
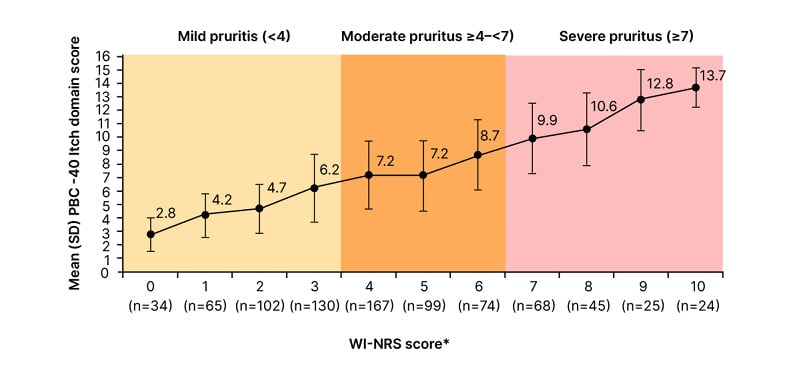
Figure 2: PBC-40 Itch domain scores increase with WI-NRS scores,* with the threshold for clinically significant pruritus on PBC-40 (≥7) corresponding to moderate-to-severe itch (≥4) on WI-NRS.18
*Responses to WI-NRS were used to calculate WIS. WIS levels were defined as: 0=0; >0–1=1; >1–2=2; >2–3=3; >3–4=4; >4–5=5; >5–6=6; >6–7=7; >7–8=8; >8–9=9; >9–10=10. The pooled timepoints included Weeks 4, 8, 12, 16, and 20 from the Phase IIb study and Day 8 from the PRO validation study. Data were pooled from 288 patients from which there were 833 observations.
WI-NRS: Worst-Itch Numerical Rating Score.
“Routine use of either the WI-NRS or PBC-40 Itch domain in clinical practice would enable identification of patients in need of pruritus management to improve their HRQoL,” concluded the researchers.19
Conclusion
The significant burden of pruritus in patients with PBC highlights an urgent need for improved management strategies.12-14,16,18,19 The current inadequacy in treating this symptom not only affects individual patients but also places a strain on healthcare systems due to increased resource utilisation.14 As research continues to demonstrate the effectiveness of validated PROs in assessing pruritus and HRQoL, it is imperative healthcare professionals incorporate these tools into routine clinical practice. By consistently evaluating itch severity and its impact on patients’ lives, clinicians can better understand the challenges people living with PBC face and tailor interventions accordingly. The findings outlined above emphasise that the specific assessment method is less critical than the commitment to regularly evaluate pruritus. This approach could facilitate timely and appropriate treatment, improving the overall QoL of patients suffering from this debilitating symptom.
NX-GBL-HP-BRFS-250001 | March 2025







