INTRODUCTION
Non-alcoholic steatohepatitis (NASH) is the advanced form of non-alcoholic fatty liver disease which can lead to further liver damage, advanced fibrosis, cirrhosis, and hepatocellular carcinoma. The mechanism for the progression of NASH involves multiple parallel pathways including oxidative stress, mitochondrial dysfunction, and inflammation.1,2
AramcholTM (arachidyl-amido cholanoic acid), a novel fatty acid bile acid conjugate, is currently being developed for the treatment of NASH and fibrosis. Aramchol is a liver-targeted, oral stearoyl coenzyme A desaturase 1 (SCD1) modulator that has been shown to reduce liver fat and fibrosis in patients with NASH in a Phase IIb clinical study (ARREST).3 Aramchol has a unique mechanism of action which targets both the metabolic alterations that characterise NASH (accumulation of lipids, lipotoxicity, and oxidative stress) and fibrosis.4 A Phase III clinical trial of Aramchol in patients with NASH and fibrosis (ARMOR) was initiated in 2019.5
This article reviews two Aramchol-related posters presented at The Liver Meeting® 2019. The first, by Trevitt et al.,6 presented clinical data on the rationale for the Phase III ARMOR trial Aramchol dose selection and its potential increased efficacy. Findings presented in the second poster by Mato et al.7 expand on previous Phase IIb data showing how Aramchol treatment resulted in a reduction of HbA1c in NASH patients compared with placebo.3 This new preclinical research elucidates the mechanism by which Aramchol regulates liver glucose metabolism, and may explain the effect of Aramchol on downregulation of HbA1c levels seen in NASH patients.
Increased Exposure of Aramchol by Using a Split Dose – Potential for Greater Efficacy in NASH
Doctor Graham Trevitt
This Phase I study assessed whether splitting an Aramchol 600 mg once daily (QD) dose into 300 mg twice daily (BID) could significantly increase plasma concentrations of the drug. The study was conducted to further investigate observations from the recently completed Phase IIb ARREST study that had reported a dose-response relationship with Aramchol in patients with NASH. The efficacy of a 600 mg QD dose of Aramchol was superior to that of 400 mg QD for all key endpoints, including NASH resolution without worsening of fibrosis and fibrosis improvement without worsening of NASH. The dose of 600 mg increased mean Aramchol exposure by just 22% compared to 400 mg, suggesting that greater exposure could further increase efficacy.3 Aramchol is a Biopharmaceuticals Classification System (BCS) Class IV drug and therefore has exposure subproportional to dose.
To overcome this common Class IV problem to increase exposure and potentially efficacy, Dr Trevitt and his team modelled pharmacokinetic (PK) data from Phase I and II studies and performed a simulation to predict the relative bioavailability, area under the curve (AUC), and minimum plasma concentration (Cmin) that would be achieved with a 300 mg dose of Aramchol administered every 12 hours, compared with these exposure parameters achieved using a dose of 600 mg QD. This predicted that 300 mg BID would produce a Cmin 1.4-fold and an AUC 1.3-fold higher than that after 600 mg QD.
The team then performed the current Phase I, two-period, open-label, randomised, crossover PK study in 16 healthy volunteers to compare plasma concentrations at steady state after oral dosing with Aramchol 300 mg BID and 600 mg QD. The subjects completed two 10-day study periods. In Period 1, eight subjects were randomised to 300 mg BID and the remaining eight to 600 mg QD. Following a minimum 3-week washout, the volunteers received the alternate treatment in Period 2. PK parameters were assessed during the dosing interval on Day 10.
The split dose regimen resulted in significantly higher exposures than QD dosing, with the average exposure being 53% higher (ratio of geometric mean values of AUC to the end of the dosing period [AUCtau] was 153 [90% confidence interval: 1.38–1.69]). All subjects showed higher values on the BID regimen (Figure 1A and B).
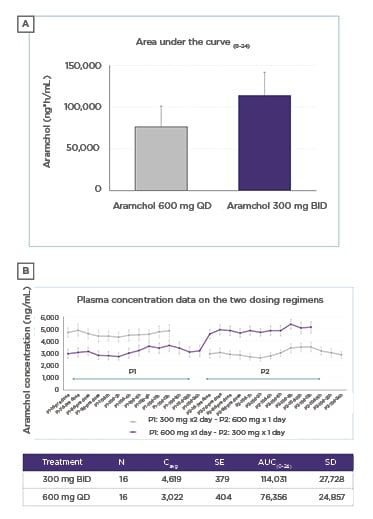
Figure 1: ‘Dose split’ method significantly increased exposure of AramcholTM compared with the same total dose taken once daily.
AUC(0–24): area under the curve during a 24-hour period; BID: twice daily; Cavg: average concentration; d: days; h: hours; P: period; QD: once daily; SD: standard deviation; SE: standard error.
The prediction from the PK model was borne out in practice with substantially higher concentrations of Aramchol obtained in human plasma with the 300 mg BID regimen compared with 600 mg QD. Dr Trevitt concluded that based on the apparent dose-response pattern observed in the Phase IIb ARREST trial (with a 22% increase in exposure), this increase in exposure (53%) offers the possibility for even greater efficacy in the treatment of NASH with Aramchol than was observed with 600 mg QD in the ARREST study. As a result, the Phase III ARMOR study will evaluate the efficacy and safety of Aramchol 300 mg BID versus placebo in patients with NASH and fibrosis Stage 2 or 3 who are overweight or obese (BMI between 25 kg/m2 and 40 kg/m2), and have prediabetes or Type 2 diabetes mellitus.5
Aramchol, a SCD1 Regulator, Improves Liver Glucose Homeostasis in NASH
Professor José M. Mato
This preclinical study explored the mechanism by which Aramchol regulates liver glucose metabolism using hepatocytes in vitro and the methionine and choline deficient (MCD) diet mouse model of NASH. The study was conducted to further investigate findings that NASH patients treated with Aramchol (400 mg/day or 600 mg/day) in the Phase IIb ARREST study had significantly better glycaemic control compared with those receiving placebo; patients in the placebo arm had increased HbA1c at Week 52, while those treated with Aramchol showed a significant reduction in HbA1c, with a dose-response pattern suggesting that Aramchol targets liver glucose metabolism.3
To further explore these findings, Prof Mato and his team treated murine primary hepatocytes with 20 µM Aramchol for 48 hours and then analysed the liver proteome and AMP-activated protein kinase (AMPK)/S6 pathway. As expected, SCD1 levels were downregulated. Increased AMPK phosphorylation and carnitine palmitoyltransferase IA/B concentrations were also detected, together with a reduction in ribosomal protein kinases (p-p70S6K, p70S6K, p-S6, and S6) and total acetyl-coenzyme A carboxylase. These results indicated stimulation of catabolic pathways and a reduction of anabolism.
Aramchol treatment targeted key biological processes; a reduction in translation and fibrosis, and activation of lipid droplet clearance, fatty acid oxidation, oxidative phosphorylation, antioxidant response, and the tricarboxylic acid (TCA) cycle was found in the analysis of 200 proteins differentially expressed between aramchol-treated and vehicle hepatocytes.
The researchers added 13C-labelled glucose to Aramchol-treated and vehicle hepatocytes to explore the identified changes in the TCA cycle. Results showed an increase in the number of rounds that malate remains in the cycle, indicating a reduction in the removal of intermediate metabolites (cataplerosis), which is linked to reduced gluconeogenesis.
The team also collected liver samples from mice fed with the MCD diet containing 0.1% methionine (0.1 MCD) for 4 weeks, including a group that were given Aramchol (5 mg/kg/day or 1 mg/kg/day) for the last 2 weeks in the treatment model, as well as from mice fed the control diet. Metabolic analyses showed that compared with mice fed the control diet, those fed the 0.1 MCD diet showed a reduction in liver glucose, glucose 6-phosphate, fructose 6-phosphate, uridine diphosphate glucose, ribulose 5-phosphate, fructose 1,6-bisphosphate, and pyruvate. Treatment with Aramchol improved glycolysis/gluconeogenesis in mice fed the 0.1 MCD diet in a dose-dependent manner, and resulted in reduced liver steatosis, fibrosis, and inflammation.
In summary, the data indicate that Aramchol stimulates glucose and fatty acid catabolism via AMPK pathway activation and reduces gluconeogenesis and lipogenesis through the mTOR pathway inhibition, resulting in improved liver glucose homeostasis. These findings show that Aramchol treatment not only improves NASH features by reducing lipid accumulation, inflammation, and fibrosis, but additionally improves liver glucose homeostasis in line with clinical data. According to Prof Mato, the new results may explain the effect of Aramchol on HbA1c levels observed in patients in the ARREST study and highlight its therapeutic value in
NASH treatment.








