Meeting Summary
Cholestatic liver diseases include primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and progressive familial intrahepatic cholestasis (PFIC). In all of these conditions, cholestatic itch is a major symptom that can severely and chronically impact a person’s quality of life (QoL). At a satellite symposium presented at the 2022 meeting of the American Association for the Study of Liver Diseases (AASLD) in Washington, D.C., USA, leading experts discussed the importance of assessing itch in all patients with one of these cholestatic liver diseases. The experts presented patient cases to illustrate the challenges of managing itch in these cholestatic liver diseases. Studies show that many of these patients are not being adequately treated for this important symptom. However, while there are several treatments for itch, although not all are specifically approved medications, finding the right one for each patient may be a process of trial and error. In some cases, for people with severe, chronic, non-treatment-responsive cholestatic itch, a liver transplant may be the only treatment option.
Introduction
When bile flow is impaired in cholestatic liver diseases, one well-documented symptom is itch, which can severely affect a patient’s QoL.1-4 Among the causes of cholestatic liver disease are the cholangiocellular cholestasis conditions PBC and PSC, and the hepatocellular cholestasis condition PFIC.5 During a satellite symposium at the 2022 meeting of the AASLD, leading experts Cynthia Levy, Schiff Center for Liver Diseases, Division of Digestive Health and Liver Diseases, University of Miami, Florida, USA; Gideon Hirschfield, Toronto Centre for Liver Disease, University of Toronto, Ontario, Canada; Andreas Kremer, Department of Gastroenterology and Hepatology, University Hospital Zurich, Switzerland; and Kidist Yimam, Department of Hepatology and Liver Transplantation California Pacific Medical Center, San Francisco, USA, provided an overview of these cholestatic liver diseases, framed within real-life patient cases to provide an understanding of the approaches to diagnosis and treatment of itch.
Cholestatic liver diseases can be diagnosed based on a combination of laboratory tests, imaging studies, genetic tests, and histopathology examination, which can distinguish between PBC, PSC, and PFIC. The majority of patients with PBC show raised serum levels of alkaline phosphatase (ALP), γ-glutamyltranspeptidase (GGT), and serum antimitochondrial antibodies ≥1:40. Patients may also have disease-specific and non-specific anti-nuclear antibodies, and elevated IgM, cholesterol, conjugated albumin, alanine transaminase, and aspartate aminotransferase.5 Histologically, PBC is graded in severity as Stages I–IV, with Stage I showing non-suppurative destructive cholangitis, Stage II with ductular reaction and periportal necroinflammatory activity, Stage III showing multiple portal-portal bridging fibrosis, and finally, Stage IV consisting of biliary cirrhosis with nodule formation.6 Ultrasound imaging will show a normal biliary tree.5
PSC can affect both extra- and intra-hepatic bile ducts with the occurrence of multifocal bile duct strictures, usually demonstrated using magnetic resonance cholangiopancreatography or endoscopic retrograde cholangiopancreatography (ERCP). Patients with PSC may present with hepatomegaly and splenomegaly. Raised ALP is common in PSC, with many, but not all, showing raised serum aminotransferases, bilirubin, and IgG. Autoantibodies may be detected in PSC, but they are not diagnostic. Liver biopsy in PSC also shows four histological stages, with a progression from portal oedema, mild chronic inflammation, and ductular proliferation in Stage I, lesion extension with interface hepatitis and periportal fibrosis in Stage II, bridging fibrous septa and degeneration of bile ducts in Stage III, then cirrhosis in Stage IV.7 While ultrasound findings in PSC may be normal, biliary tree changes, including strictures and a beaded pattern, may be revealed by cholangiography.8
PFIC is a rare autosomal recessive disease with a number of genetic phenotypes. PFIC Types 1–6 are distinguished by mutations in the ATP8B1, ABCB11, ABCB4, TJP2, NRIH4, and MYO5B genes, respectively.9 PFIC typically presents in infancy or childhood. In PFIC1, while bilirubin, serum transaminases, and bile acids will be elevated, GGT is low. The liver biopsy in patients with PFIC1 shows fibrosis, but bile duct changes may not be present. Most patients with PFIC1 develop end-stage liver disease in childhood.5 Progressive liver disease also develops in patients with PFIC2, which has the histological characteristic of giant cell hepatitis and portal inflammation, with low levels of GGT.5,10 Patients with PFIC2 may develop hepatocellular carcinoma or cholangiocarcinoma.11 Although PFIC3 can also present in childhood, in some patients, it may not manifest clinically until adulthood. In PFIC3, GGT is typically elevated and there is progressive cholestasis and cirrhosis, as well as large bile duct proliferation, and, in some cases, intrahepatic gallstone disease.12 These patients are at an increased risk for cholelithiasis, intrahepatic cholestasis of pregnancy, and oral contraceptive-induced liver injury. PFIC3 is also associated with increased risk of cholangiocarcinoma.9
The goal of treatment for people with cholestatic liver disease is to prevent end-stage liver disease and manage any symptoms associated with liver damage, including inflammation, fibrosis, bile duct loss, and lymphocytic cholangitis.13 For patients with PBC, clinical management includes first-line therapy with ursodeoxycholic acid (UDCA; 13–15 mg/kg/day) and second-line therapy with obeticholic acid (OCA; 5–10 mg/day). Fibrates may also be used, although these are off-label for PBC.14,15 Similar to PBC, PSC therapy may include UDCA, used off-label.5 UDCA may relieve some symptoms for patients with PFIC3, and for children with PFIC1 and PFIC2, partial external biliary diversion and terminal ileal exclusion may be effective. Liver transplantation is an option for patients with late-stage cholestatic liver disease, both due to liver disease and for severe, treatment-resistant itch.5,15,16
Case Study 1: Primary Biliary Cholangitis
Gideon Hirschfield
A 44-year old female with a history of smoking, and whose mother has PBC, presented with symptoms including significant itch, lethargy, brain fog, and fatigue. Diagnostic tests (Table 1) revealed significantly elevated transaminases, cholestasis, indications of an inflammatory process involving the bile ducts, and the presence of ductopenia and early jaundice with liver heterogeneity on imaging, indicating potential fatty liver or early fibrosis. The spleen size was normal. The focus for patient management was on both the symptoms and the patient’s QoL.
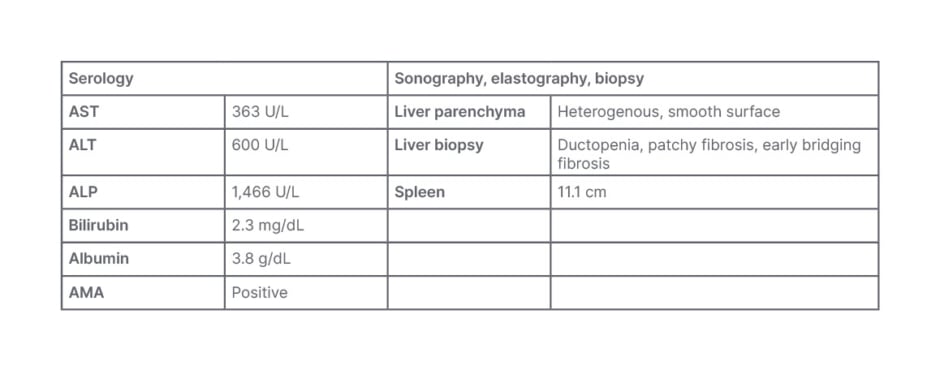
Table 1: Serology for Case Study 1.
ALP: alkaline phosphatase; ALT: alanine transaminase; AMA: anti-mitochondrial antibodies; AST: aspartate aminotransferase.
Treatment Course and Itch
The patient was initially treated with UDCA and anion-exchange resins, and their level of itch was moderate. Six months later, they were additionally prescribed anion-exchange resins, OCA (AASLD guidelines recommend OCA at 1 year), and rifampicin. As a result, their ALP/alanine transaminase (ALT) levels reduced by approximately 50% from baseline; however, the level of itch was severe. Their treatment was altered 1 year later to include UDCA, OCA, and fibrates; however, again, while their ALP/ALT levels reduced by approximately 80% from baseline, their level of itch was still severe.
Two months after this treatment change, the patient underwent several tests, including a FibroScan (Echosens, Paris, France) for liver fibrosis (8.8 kPa) and a liver biopsy, which revealed 50% duct loss. As a result, they were diagnosed with advanced stage PBC (ductopenia, focal bridging fibrosis). With continued treatment, their ALP levels fell to below 100 U/L, but their ALT levels were slightly rising and the itching remained severe. A year later, their treatment was altered again to include UDCA, OCA, fibrates, and rifampicin. While their ALP levels remained below 100 U/L, the ALT levels continued rising slightly rising. Their level of itch was classed as moderate.
The take-home message from this case is that even with maximal medical treatment for PBC and itch, the patient still has the burden of itch and they report chronic QoL issues. Therefore, there is still room for improvement.
Clinically Significant Itch in Cholestatic Liver Disease
“We need to pay attention to our patients,” said Levy about itch in cholestatic liver disease. “We need a more patient-centric approach.” Hirschfield confirmed this, adding: “It is important that you understand patient priorities as well as disease control priorities.” Studies have shown that approximately 81% of people with PBC, between 38−65% of people with PSC, and up to 80% of people with PFIC experience itch.2,3,17,18 In some cases this can be clinically significant and severe, as was shown in these studies in 37% of the PBC2 and all the PFIC cases.3 During the symposium, patient testimonies, as captured in Figure 1, revealed their experience of itch, and the impact it has on their QoL.

Figure 1: Patient comments regarding their experience with itch associated with cholestatic liver disease.
These testimonies are supported by a study of patients with PBC who described the symptoms of cholestatic itch as feeling like ‘bugs crawling’ on their skin, the sensation of ‘burning’, with a ‘prickly’ feeling, like ‘needles’.1 Patients also described that the itch was ‘deep and relentless’, could be so severe that it made them ‘want to tear my skin off’, and that the itch made them ‘itch until I bleed’.1 Of note, in cholestatic itch, in general scratching the itch does not bring symptomatic relief.19
Importantly, itch can impact the QoL of patients and their caregivers.2-4,20 This is not just through the experience of itch itself, and subsequent abrasions and scarring from itching, but also by disrupting sleep and contributing to fatigue, as well as being associated with social and emotional stressors such as isolation and depression.2-4 “Relentless itch will drive anyone’s tolerability down and their ability to cope, their fatigue, their ability to have relationships and go to work… is not very high if you’re chronically itchy,” said Hirschfield.
Real-world patient histories and the findings from studies on itch in cholestatic liver disease are supported by the USA-based 5-year, longitudinal, observational study, TARGET-PBC.2 This study included 211 patients with PBC.2 Compared with patients with mild or no itch, patients with a clinically significant itch had significantly higher scores in fatigue, cognitive, emotional, and social impairment domains.2 One UK study evaluated 2,194 patients with PBC and found that for 35% of patients, itch was persistent. Of those who reported itch, 54% had low itch, 28% had mild itch, and 18% had high itch. In all three groups, while intensity of itch changed moderately over a period of 6 years, presence of itch was constant.21 Interestingly, evidence of whether itch is directly correlated with the stage or duration of PBC disease, or serological findings, is inconsistent.13,15,21-23 Some show no significant correlations, while others show a correlation between clinically significant or persistent itch, and high alkaline phosphatase.22
Case Study 2: Progressive Familial Intrahepatic Cholestasis
Andreas Kremer
This case study involved a 34-year-old female, 1.58 m tall, weighing 55 kg with a BMI of 22.0, who was physically active, had a leading position in a technology company, was married, and had a 6-year-old child. The patient was referred by their general practitioner for a second opinion. They were diagnosed with antimitochondrial antibodies-negative PBC (liver biopsy 2008; anti-M9 temporarily increased), had a cholecystectomy in 2011 due to cholecystitis with stones, and had Hashimoto thyroiditis. On referral, they had scleral icterus for the past 4 weeks.
Symptoms included moderate itch (average numeric rating scale [NRS]: 6/10), which started when they were approximately 15 years old. The itch disturbs their sleep and, while they can work during the week, they are fatigued, and it impacts their weekends and time with their partner and child.
Their current treatment includes UDCA (750 mg/day), L-thyroxin (100 µg/day), an oral contraceptive, and oral antihistamines for itch.
The serology results (Table 2) showed a cholestasis-driven picture with increased ALP, bilirubin, and fasting total bile acids. There was no indication of autoimmune hepatitis or HIV infection. Sonography results excluded obstructive cholestasis but showed an irregular liver surface and large spleen, pointing, with transient elastography results, to advanced liver disease. Genetic sequencing identified mutations in the ABAB4 gene, and predisposing mutations in ABCB4 and ABCB11 genes, indicative of PFIC3.
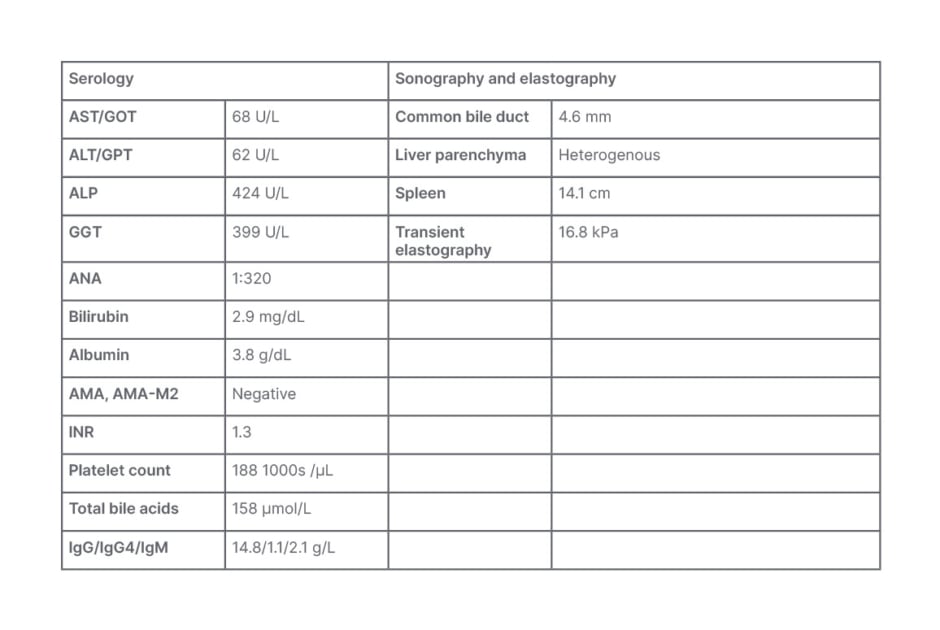
Table 2: Serology for Case Study 2.
ALP: alkaline phosphatase; ALT: alanine transaminase; AMA: anti-mitochondrial antibodies; AMA-M2: anti-mitochondrial M2 antibody; ANA: antinuclear antibodies; AST: aspartate aminotransferase; GGT: gamma glutamyl transferase; GOT: glutamic oxaloacetic transaminase; GPT: glutamic-pyruvic transaminase; IgG: immunoglobin G; IgG4: immunoglobin G4; IgM: immunoglobin M; INR: international normalised ratio.
Itch Treatment
Itch progressed in intensity (NRS: 8−9/10) and how much it affected the patient’s QoL. There was no benefit from antihistamines and an anion-exchange resin was not tolerated due to the unpleasant taste, so it was terminated by the patient. Rifampicin was not prescribed due to drug-drug interactions with oral contraceptives, which the patient wished to remain taking, and a fibrate brought mild benefit but was not tolerated due to myopathy. The patient discussed how they were afraid of not being able to function sufficiently in their job. They were treated with an opioid receptor antagonist, then a selective serotonin uptake inhibitor (SSRI), but without any benefit. The patient was prescribed odevixibat in May 2022 with a modest initial benefit (NRS 5/10) and then a more significant benefit with a dose increase in August 2022 (NRS 2/10). There was a mild increase of bowel movement rate to twice a day. Sleep and QoL improved, as did liver function tests, with a lowering of total serum bile acids from 34 µmol/L.
The take-home message from this case is that it is important to inform the patient that it may take several treatment options to find the right treatment to reduce their itch.
Assessment of Cholestatic Itch
Hirschfield discussed the importance of a correct diagnosis of cholestatic itch, and relayed how some patients may be incorrectly referred to a dermatologist instead of a hepatologist. This, Hirschfield reported, could result in long delays before initiating proper treatment.
There are some simple ways to assess itch, highlighted Levy; however, they stressed the importance of not making assumptions regarding the occurrence and level of itch, and asking the patient instead of guessing these factors, as studies have indicated that clinicians often underestimate the intensity of itching unless it is very severe.24 Assessment tools include an NRS or verbal rating scale from 0 (no itch) to 10 (worst imaginable itch); and the single-item, self-reported categorical Patient Global Impression of Severity (PGI-S) scale that rates itch as absent, mild, moderate, severe, or very severe. 21,25 Of note though, said Yimam, these current scales are not specific for cholestatic itch and do not assess QoL.
To gain a complete picture of the effects of cholestatic itch, Kremer highlighted that the patient should be asked about both average itch and worse itch, not just what they are experiencing at the time of a consult. Levy also suggested that there are smartphone or computer applications the patient can use to rate symptoms. For children with PFIC, discussed Kremer, it is important to also gain the caregiver’s perspective, both via filling out rating scales and through discussion of how the experience of itch affects the child’s QoL by, for example, disrupting their sleep.
Hirschfield discussed how, for PBC, a ‘traffic light evaluation’ method of triaging takes into account age, bilirubin, and ALP levels. Here, those considered high risk (≤50 years; bilirubin >1x upper limit of normal; ALP >3x upper limit of normal), who also have the lowest chance of 1-year transplant-free survival, require an enhanced PBC-dedicated programme.26 However, Hirschfeld discussed how decision-making regarding this pathway should also take into account the impact of symptoms, including itch and fatigue.
Treatment for Cholestatic Itch
Although there are a few treatment options for itch in cholestatic liver disease, Levy discussed how there is an unmet need for more effective disease management strategies. For example, the TARGET-PBC study found that only a third of patients with clinically significant itch received treatment, and of those, only a quarter received guideline-recommended first-line therapy.2 Similarly, the findings from a UK study showed that only 67% of 1,805 patients who reported cholestatic itch received treatment.27
Itch treatment should include practical recommendations such as avoiding heat, including hot water, tight or itchy clothing, and avoiding emollients.15,16,28 Current medications include anion exchange resins (e.g., cholestyramine), usually as the first-line choice; opioid receptor antagonists (e.g., naltrexone); pregnane X receptor agonists (e.g., rifampicin); fibrates (e.g., bezafibrate); antihistamines; SSRIs; and, in PFIC, ileal bile acid transporter inhibitors (e.g., odevixibat). However, clinical management guidelines regarding the choice of treatment may differ between types of cholestatic liver disease and what has been approved for each condition.15,16,29-31
Furthermore, several potential side effects of these medications need to be discussed with the patient. For example, renal insufficiency, myalgia, and hepatotoxicity have been reported with bezafibrate; hepatitis can be a side effect of rifampicin; opioid withdrawal syndrome can occur with naltrexone; abdominal discomfort can be associated with anion-exchange resins, and diarrhoea with ileal bile acid transporter inhibitors.30,32
Yimam reported the findings from a small trial of a fibrate, the FITCH trial.33 This study included 74 patients with PBC or PSC and moderate to severe itch, of whom half received 400 mg/day bezafibrate and half received a placebo. After 21 days, while 41% of 46 patients with PSC and 55% of 26 patients with PBC had a ≥50% reduction in the visual analogue scale score, this only occurred in 11% and 13%, respectively, of patients receiving the placebo. Once bezafibrate was discontinued, itch scores returned to baseline at Day 35 follow-up. No major side effects were observed during the 21 days of treatment.33 Bezafibrate is not currently licensed for PSC, PBC, or cholestatic itch, and is not currently available in the USA.
Treatment options for refractory itch include surgical biliary diversion, partial external biliary diversion, and liver transplantation.5,15,16,30 Other therapies include plasmapheresis and phototherapy, which, for PSC, are considered third-line treatment options.30 There are also ongoing clinical trials aimed at treating itch for patients whose QoL is considerably impacted by cholestatic itch.
Case Study 3: Primary Sclerosing Cholangitis
Kidist Yimam
A 26-year-old male patient, previously healthy, who was a computer programmer, reported itch for a few months to their primary care provider. It mostly affected their legs and feet, and prevented them from getting a good night’s sleep and concentrating at work. The itch impacted the patient’s QoL, they were left feeling fatigued and irritable, and were stressed about falling behind on projects at work. The patient tried over-the-counter systemic antihistamines without a lasting benefit, and stopped taking them as they made them feel sleepy at work.
The patient reported intermittent right upper quadrant abdominal pain without nausea, vomiting, fever, or chills. They did not have diarrhoea or blood in their stool. Initial serology by the primary care provider (Table 3) showed elevated ALP and aspartate aminotransferase. Their liver was normal on imaging.
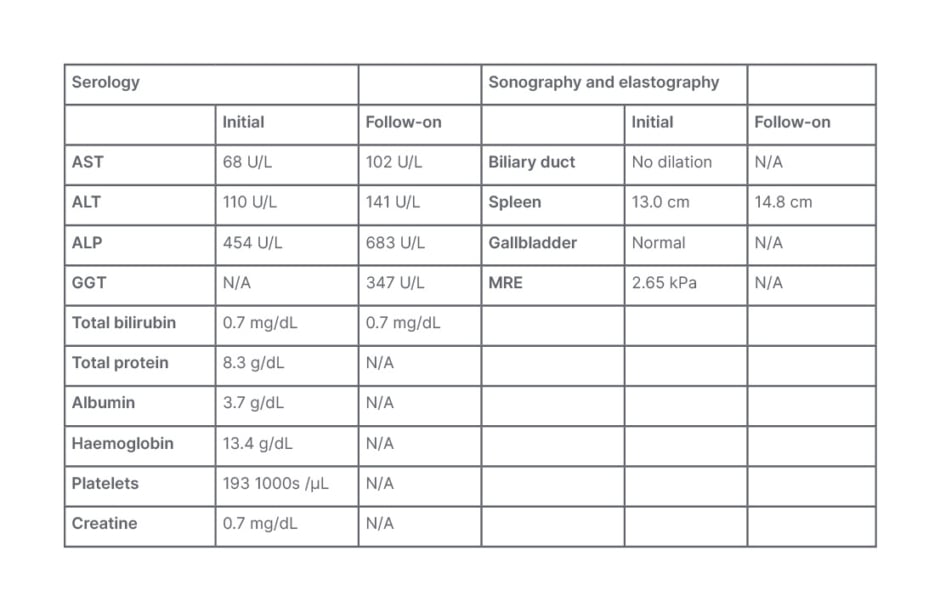
Table 3: Serology for Case Study 3.
ALP: alkaline phosphatase; ALT: alanine transaminase; AST: aspartate aminotransferase;
GGT: gamma-glutamyl transferase; MRE: magnetic resonance elastography; N/A: not applicable.
On referral to a hepatology clinic for follow-on (Table 3), perinuclear anti-neutrophil cytoplasmic antibodies were positive, IgG4 was normal, and colonoscopy identified a mild right-side chronic colitis. A CT scan showed a mildly enlarged spleen. Also found were irregularities of the common hepatic duct and left and right hepatic ducts, with beaded appearance of the intrahepatic bile ducts. They did not have advanced liver fibrosis. These results supported a diagnosis of PSC.
Itch Treatment
The patient was prescribed a different systemic antihistamine with mild benefit and an anion-exchange resin that caused abdominal pain and dyspepsia, so they stopped taking it. They underwent ERCP, which showed that they did not have a dominant biliary stricture to dilate. They were prescribed naltrexone with no significant benefit. While offered rifampicin, the patient was concerned about potential hepatoxicity, so they declined. They were prescribed a daily SSRI and anion-exchange resin, and experienced mild-moderate improvement; however, the itch remained at the moderate and sometimes severe level, and it was still significantly affecting their QoL.
The patient was enrolled in a PSC disease-modifying clinical trial, and the itch worsened. The study was terminated for independent reasons. Two years after their initial treatment, they underwent another ERCP to evaluate and treat biliary obstruction, with mild benefit for the symptoms of itch.
The take-home message is that if after several treatments, itch still has a significant impact on the patient’s QoL, third-line treatment options and liver transplantation could be considered.
Conclusion
In concluding the symposium, Levy stressed the need to make the correct diagnosis of cholestatic liver disease initially and, if necessary, to include genetic testing as part of the clinical diagnostic work-up. The panel highlighted how patients need to be listened to, and have their itch assessed with an NRS or visual analogue scale with descriptions of their experiences and how itch affects their QoL. Levy concluded by stating the importance of informing the patient that the journey to find the best treatment for their itch may be timely, involving a trial and error investigation of different treatment options over several weeks to months.