Meeting Summary
In the progressive, immune-mediated liver disease primary biliary cholangitis (PBC), the intrahepatic bile ducts are gradually destroyed over several years. The primary biochemical means to diagnose PBC, and assess progression and treatment response, is serum alkaline phosphatase (ALP). Further diagnostic criteria depend on antimitochondrial antibody (AMA) and specific antinuclear antibody status, and histological confirmation in some patients. First-line therapy for PBC is ursodeoxycholic acid (UDCA), which has been shown to improve biochemical indices of PBC and slow disease progression. However, major quality of life (QoL)-impacting symptoms of PBC, including pruritus and fatigue, are demonstrated to be independent of disease severity. There is evidence confirming that these symptoms negatively impact a number of aspects, including emotional status, ability to work, and social life, for some patients. In a symposium as part of the European Association for the Study of the Liver (EASL) International Liver Congress™ (ILC) 2022, Gideon Hirschfield, Toronto Centre for Liver Disease, University of Toronto, Ontario, Canada; Ana Lleo, Humanitas University and Humanitas Clinical and Research Centre, Milan, Italy; and David Jones, Newcastle University and Newcastle-upon-Tyne Hospitals NHS Foundation Trust, UK, discussed the holistic treatment of patients with PBC and whether goals of such should be more or equally dependent on biochemical status or impact on QoL. This discussion was expanded on in a session moderated by Jessica K. Dyson, Newcastle University and Newcastle-upon-Tyne Hospitals NHS Foundation Trust, UK.
Introduction
PBC is an immune-mediated, chronic, progressive liver disease characterised by gradual destruction of intrahepatic bile ducts. Reflecting this, alongside cholestasis is serologic reactivity to highly-specific antinuclear antibody or AMA.1,2 PBC more commonly occurs in females and people can live with PBC for several decades.3-5
Current EASL treatment guidelines recommend that PBC be stratified by disease stage and risk of liver-related complications, including death. In patients with no alternative explanation for abnormal liver blood tests, guidelines advise that ‘probable’ PBC can be diagnosed on the basis of elevated serum ALP and AMA at a titre >1:40, negating the need for liver biopsy in many patients.1
In patients who are AMA-negative with biochemical abnormalities, the diagnosis of PBC requires histological confirmation. The EASL guidelines recommend a liver biopsy if PBC-specific antibodies are absent, or if there is diagnostic uncertainty, such as the possibility of concomitant autoimmune hepatitis. If a patient has normal liver blood tests but is AMA-positive, clinical practice often consists of annual review to assess for the development of biochemical abnormality.1
In a symposium as part of the EASL ILC, held between 22nd and 26th June 2022 in London, UK, and online, Hirschfield discussed the possibilities of holistically treating patients with PBC, Lleo asked if the focus of treatment goals should be on ALP, Jones counterquestioned whether the focus should be on managing QoL factors, and Dyson moderated a question and answer session.
Treatment for Primary Biliary Cholangitis
PBC therapy aims to reduce morbidity, mortality, and symptom burden.1 The only fully approved first-line medication for PBC is UDCA, using a therapeutic dose of 13−15 mg/kg/day, which should be initiated for all patients with PBC. For patients with an inadequate response to, or who are intolerant of, UDCA, obeticholic acid is approved as an adjuvant second-line therapy or monotherapy.1,6 Also, for patients with inadequate UDCA response, the American Association for the Study of Liver Diseases (AASLD) recommends the off-label use of fibrates.6 These are not currently recommended in EASL guidelines due to the lack of evidence from Phase III randomised clinical trials.1 However, several studies have shown their utility with regard to biomarker response,7 PBC progression,8 and positive effect on pruritis9 when combined with UDCA or as monotherapy. The use of obeticholic acid and fibrates is discouraged by the AASLD in patients with decompensated liver disease (Child–Pugh–Turcotte B or C).6
UDCA has been shown to improve biochemistry and long-term outcomes with a reduced need for liver transplantation and death. For instance, in one study, 90% of 4,119 UDCA-treated patients showed a 5-year transplantation-free survival rate, with rates of 78% at 10 years and 66% at 15 years. Respective survival rates in 726 non-UDCA treated patients were significantly lower: 79%, 59%, and 32%, respectively (UDCA-treated versus nontreated patients, p<0.0001).10 UDCA has also been shown to improve the outcomes of biochemical liver tests, including ALP, bilirubin, and aminotransferases.11 Of note though, depending on the criteria used (Table 1), inadequate response to UDCA was shown in 25−50% of patients.17,19
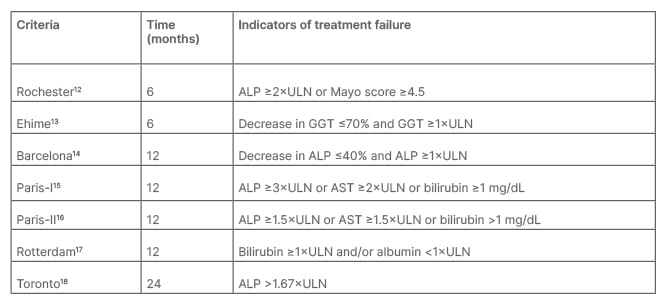
Table 1: Criteria for assessing biochemical response in primary biliary cholangitis.1
ALP: alkaline phosphatase; AST: aspartate aminotransferase; GGT: γ-glutamyltranspeptidase; ULN: upper limit of normal.
Monitoring Primary Biliary Cholangitis Progression and Treatment Response
EASL guidelines recommend monitoring response to UDCA and disease progression using biochemical means, including ALP, transaminases, and bilirubin. Response can be assessed using quantitative definitions with discrete, binary criteria or quantitative continuous scoring systems. As shown in Table 1, there are several qualitative binary criteria, including assessment of treatment response at 6, 12, and 24 months.1 “It doesn’t really matter which criteria you use,” remarked Lleo, “the important concept is that you need to assess if the ALP of your patient has been reduced compared to baseline.”
Although these criteria include biochemical levels predominantly still above the upper limit of normal (ULN), Lleo suggested that “it’s not enough to have an ALP that is below 1.5; we should aim for normal ALP as these have been associated with better survival, and this is important when we need to evaluate if our patient is a candidate for second-line treatment.” A study of 2,555 patients treated with UCDA showed that any increase in bilirubin or ALP above ULN at Year 1 was associated with an increased risk for liver transplantation or death. Moreover, a stable decrease in bilirubin to <0.63xULN was associated with an 11% improvement in 10-year survival or liver transplantation. In people with an ALP <1xULN, 10-year survival was 93.2% compared with 86.1% with ALP 1.0–1.67xULN.20
Approximately 8−10% of patients with PBC present with a variant that is sometimes termed PBC-autoimmune hepatitis overlap. Here, the Paris diagnostic criteria include at least two of ALP >2xULN or γ-glutamyltranspeptidase >5xULN, AMA >1:40, and florid bile duct lesion, as well as two of alanine aminotransferase >5xULN, serum IgG >2xULN or smooth muscle autoantibody-positive, and moderate–severe interface hepatitis on histology.21 In these cases, liver biopsy is considered mandatory.1 In patients with a true overlap syndrome, adding immunosuppressants to UDCA therapy may be beneficial.1
Continuous scoring systems can also help stratify disease risk and predict transplantation-free survival. The GLOBE score is a validated risk assessment tool specific to UDCA-treated patients that, according to Lleo, provides a reliable estimate of prognosis. The risk score includes Year 1 scores for ALP, total bilirubin, serum albumin, and platelets, and also considers age at therapy initiation.6,19,22,23 The study that helped define this score showed that patients with risk scores >0.30 had significantly shorter times of transplant-free survival than matched healthy controls.19 The GLOBE score is of particular use, said Lleo, as the biomarkers used should be universally available.
The UK-PBC score is another validated risk assessment tool for UDCA-treated patients. It can help predict liver transplant or liver-related death occurring within 5, 10, or 15 years.6,24 Serum albumin, ALP, platelet count, alanine aminotransferase, aspartate aminotransferase, and total bilirubin scores can be used to identify patients who would benefit most from further risk reduction with second-line therapy.24
Additionally, transient elastography (TE) tools, such as FibroScan® (EchoSens, Paris, France), are noninvasive means to assess liver fibrosis and cirrhosis via measurement of the degree of liver stiffness. Such tools can provide a high degree of accuracy in patients with PBC for diagnosis of advanced fibrosis, which is important as liver stiffness progression is predictive of poor outcome.25,26 A recent study utilising vibration-controlled TE found that a liver stiffness measurement cut-off of ≤6.5 kPa could diagnose the absence and >11.0 kPa the presence of advanced fibrosis, with a negative predictive value of 0.94, positive predictive value of 0.93, and error rate of 5.6%.27 TE, discussed Lleo, “is an easy to obtain exam, you can repeat it over time, and the variation can give you an idea of the prognosis of your patient.”
Several other indices have also been used to help predict outcomes in PBC. For instance, in patients without cirrhosis, according to the AASLD guidelines, ductopenia and inflammation severity are strongly related to ALP elevation, ductopenia and biliary piecemeal necrosis severity are related to hyperbilirubinaemia, and lobular necrosis and inflammation are reflected by increases in aminotransferase activity and IgG levels. Early indicators of cirrhosis and portal hypertension development include increased serum bilirubin, hyaluronic acid, and globulins, together with decreased serum albumin and platelet counts.6,28,29
As hyperbilirubinaemia is only seen in the late stages of PBC, bilirubin levels are considered less discriminatory for the detection of early disease progression and clinical outcome prediction.23,30 However, some data suggest that any increase in bilirubin level, even within the normal range, should highlight high-risk patients with prompt consideration for optimal management, including potentially second-line therapies.23
Quality Of Life in Patients with Primary Biliary Cholangitis
“Biochemical liver tests can help healthcare professionals identify the best therapy option for patients with PBC,” said Hirschfield. “However, biochemistry will not necessarily be tackling symptom burden. We think about PBC very clinically,” he continued, “but our patients have to live with it every day.” Jones agreed that “both prognosis and symptoms are equally important. However, although we talk about symptoms in practice, our patients feel that we perhaps don’t do as much as we could.”
In patients with PBC presenting moderate–severe symptoms, impacts include pruritus, fatigue, cognitive problems, social impairment, and emotional dysfunction.31 Jones explained that “when you have one bad symptom with PBC, you tend to have several of them.” These domains can be assessed using the PBC-40 QoL, a six domain, 40 question instrument that asks patients to rate the frequency of factors associated with symptoms (e.g., I had dry eyes), itch (e.g., itching disturbed my sleep), fatigue (e.g., I felt worn out), and cognition (e.g., I found it difficult to concentrate on anything), and how much they are bothered by social (e.g., I can’t plan holidays because of having PBC) and emotional (e.g., having PBC gets me down) factors.32
Cholestatic Pruritus
Cholestatic pruritus is one of the major symptoms of PBC,6 with around half of all patients experiencing this to some degree at any given time.33-35 One survey of 238 patients showed that approximately 70% of respondents experienced this at any time,36 with another survey, including 2,194 patients, showing similar rates of 73.5%.37 As PBC is a chronic disease, pruritus can persist for years, with 34.5% of patients reporting persistent pruritus over the survey period of 8 years and 11.7% of patients reporting this pruritus was severe.37
Pruritus can impact all domains on the PBC-40, which can be increased relative to the degree of itching.38 “If you itch all the time,” added Hirschfield, “you don’t sleep, you can’t control your executive functions and how can you cope with life and work, and all the things you’re trying to balance in addition to your health?” Patients also reported that scratching due to cholestatic pruritus can impact their self-esteem39 and contribute to social isolation.40 Further, the GLIMMER study of 147 predominantly female (94%) patients with PBC who also experienced severe pruritus compared administration of linerixibat (an ileal bile acid transporter inhibitor) or a placebo.41 As seen in [hll]Figure 1[/hl], analysis of participants at baseline found that health utility value (EuroQol 5-dimension measure of health-related QoL) scores42 were similar to people with severe Parkinson’s disease,43 and lower than people with heart failure,44 end-stage renal disease,45 and chronic obstructive pulmonary disorder.46 “These are catastrophically bad levels of QoL,” said Jones.
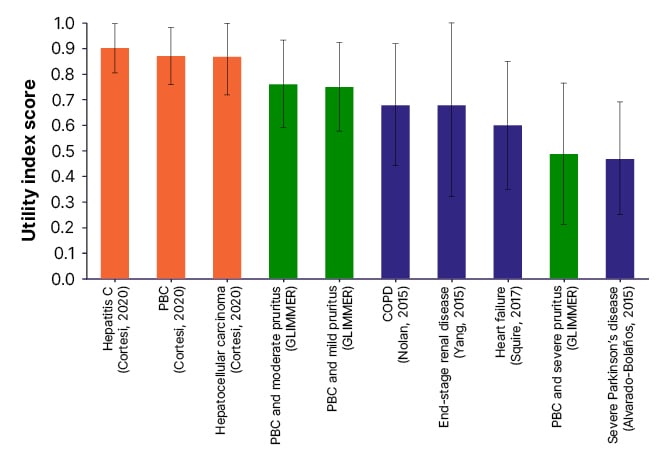
Figure 1: Health utility mean (standard deviation) of patients with cholestatic pruritus compared with liver-related and other conditions.42-47
Reproduced with permission from Smith et al.42
COPD: chronic obstructive pulmonary disorder; PBC: primary biliary cholangitis.
In a survey of 281 people with PBC, respondents were asked to confirm descriptors of pruritus. Here, 35.0% agreed it was like ‘bugs crawling’, 29.2% said the pruritus was ‘deep and relentless’, 17.6% said it made them ‘want to tear my skin off’, and 15.2% agreed that it was ‘prickly/like needles’.36 Interviews with patients with PBC presented as part of this symposium asked patients to describe pruritus in their own words. Descriptions included “it’s relentless […] it’s like a life sentence of just being uncomfortable,” “it starts to mess with your head mentally. You start thinking of ways to escape it, but you can’t,” and “I’ve gotten to the point of bleeding that it is so itchy.”48
Despite these findings, Jones reported that “people struggle to get their clinicians to take it seriously. Clinicians think that if the liver biochemistry is good, then perhaps pruritus cannot be anything to do with the liver disease.” Indeed, some studies have not shown a correlation between pruritus and biological indicators of PBC severity.49 However, others have shown that severe itch occurs more frequently in patients with cirrhosis and higher ALP levels.50 These studies also found pruritus was more common in younger patients,37,50 with other studies showing pruritus may wane with disease progression.6 As such, Jones advised that “if you do get control of the itch with improved biochemical tests, it’s a bonus, but do not assume it will happen.”
According to Hirschfield, “there is an imbalance in the consultation between the patient and the doctor.” This was reflected in a patient survey where 69.8% said their doctor had not evaluated them for pruritus.36 Reasons for this lack of evaluation may not only be because pruritus is not necessarily related to disease stage,51 but also because pruritus can be difficult to quantify as there is considerable inter- and intraindividual variation and severity can fluctuate.52 Lleo pointed out that “itching is not easy to treat, so sometimes physicians get discouraged and they don’t want to ask because they don’t want to deal with the problem.”
With this in mind, Jones emphasised that every patient with PBC should be asked at every clinic visit whether or not they have pruritus and should be educated about the connection between the two. However, he said, “don’t over-elicit these symptoms because if you push too hard, everyone in life has pruritus. You’re trying to understand the nature of the impact. Don’t just ask, ‘have you got the itch?’ because people will say yes, but often they are not troubled by it. Instead, ask, ‘have you got an itch that’s bad enough to need treatment?’”
Pruritus scores can be used to assess severity (e.g., on a 1−10 scale from minimum to maximum) and impact on QoL. Body location should be noted, as well as descriptions of how pruritus feels. Specific questions can also be asked regarding factors that may exacerbate itch, whether it is worse at any particular time of day, and whether any medications are taken to relieve the pruritus.48
Addressing pruritus symptoms is essential, highlighted Jones, because “if you can roll the itch back, you can start to roll back several other difficult symptoms.” Although there are some general medications that can help pruritis, specific treatment for PBC-related pruritus is not well developed and bile acid-binding resins are the only licenced therapy for such.1 While UDCA can improve liver biochemistry measures and histological progression, it does not significantly improve PBC symptoms, including pruritus in many cases.1,11 EASL guidelines discuss several off-label treatments, including rifampicin, opiate antagonists, selective serotonin uptake inhibitors, and gabapentin.1
Jones discussed how patients should be educated regarding lifestyle measures that might help pruritus. These are reflected in EASL guidelines and include bathing in tepid water, using ice packs, applying moisturisers and oatmeal extract, and avoiding tight or sticky clothing.1 However, in the above-mentioned survey, respondents reported that while for 65% applying something cool to the skin helped pruritus, only 14% said it was relieved by scratching, and 22% said that nothing relieved it.36
Fatigue In Primary Biliary Cholangitis
Fatigue as a symptom of PBC can also greatly impact a patient’s QoL,6,53,54 with one study showing this occurred in 68.0% of patients.53 This is an important symptom to recognise, as, according to Hirschfield, it can significantly affect wellbeing. For instance, studies have shown that fatigue in PBC is associated with diminished emotional reactions, social isolation, a lack of mobility,53 and excessive daytime somnolence.55 Fatigue is also linked to incidences of depression56 and cited as having a negative influence on family life and work.4
In a published testimony, one patient with PBC recounted how their fatigue was “mind-numbing,” and left them feeling like they were “in a fog,” and could “hardly lift one foot in front of the other,” making everything difficult. The patient also discussed that one of the problems with fatigue is that “it is hidden. I don’t look different from other people.” The patient recalled how “when I say [to people] I am tired, they tell me how tired they are, and if I try to explain the difference they do not understand what I am talking about.”57
“It’s important to quantify fatigue,” said Jones, “as it’s more of a difficult concept for people to understand.” His clinic assesses fatigue at each appointment so they can track it. There are several simple assessment tools to do this, including the Fatigue Severity Score56 and a Visual Analogue Scale.58 The Fatigue domain of the PBC-40 assessment tool can also be used. This asks a person to rate how often (never, rarely, sometimes, most of the time, or always) they feel worn out, how much fatigue interferes with their daily routine, how often fatigue ‘just hit me’, and how often PBC ‘drained every ounce of energy out of me’.32,59
Jones recommended that fatigue is discussed with patients at every clinic visit. It is crucial, he stressed, that it is explained that fatigue is a common symptom of PBC. He emphasised that people may feel uncomfortable discussing fatigue and not mention it without prompting. “Try to avoid abstract concepts,” said Jones, “like asking how bad is your fatigue as it depends on what you mean by fatigue. Talk about the things they struggle with like can they do the shopping, go out to work, or socialise with friends.” Jones also discussed how it is essential for patients to identify natural fluctuations in energy levels each day and ‘listen to their body’, and emphasised the importance of maintaining social structures.
Recommendations concerning managing fatigue include drawing up an energy-management plan and encouraging energy pacing to help conserve energy for important tasks. Patients may also be referred to a fatigue management specialist, such as an occupational or physical therapist, and patient support groups.60 Additionally, there are smartphone applications available from the PBC Foundation, of which Jones is a trustee, where patients can view the latest research information, take part in surveys, and access self-management tools.61
Once a management plan is in place, Jones encouraged people to “look for change over a long time frame, you’re not going to make people better next week or the week after but Christmas to Christmas, birthday to birthday, you’re looking for a pattern of improvement over time.”
As discussed by Jones, treating comorbidities and factors contributing to fatigue may also help lessen the symptom and its impact. “These are all things that don’t cause PBC fatigue, but they cause fatigue in the sort of people that get PBC.” For instance, treating depression, sicca syndrome, obstructive sleep apnoea, restless leg syndrome, vitamin D deficiency, and autonomic dysfunction if present.62
Conclusions
The primary therapy for PBC is UDCA, the biochemical response to which can be monitored using liver blood tests such as ALP. Such tests can be used to assess response to treatment and risk stratify patients, improving identification of patients who may benefit from second-line therapies.
A patient-centred approach to PBC requires symptom assessment and treatment rather than focusing only on biochemical indices, particularly given that symptom burden does not correlate with disease severity. Pruritis and fatigue are two common PBC symptoms that significantly impact patient QoL and are under-recognised by physicians. When PBC is accompanied by severe pruritus, health-related QoL can be as negatively impacted as people with severe Parkinson’s disease. These symptoms can significantly affect a patient’s emotional health and lead to embarrassment and social isolation. As such, recognition and active management of pruritus and fatigue are vital for patients with PBC.








