Abstract
Peginterferon α-2b (Peg IFNα-2b) continues to be used for treating a selected subset of patients with hepatitis B, in the era of direct-acting antivirals for hepatitis C infection. The authors hereby report four cases of tongue pigmentation among 21 patients with hepatitis B, whom they treated in the last 5 years, where at least 48 weeks of Peg IFN were administered. The lesions were mostly self-limiting, having no temporal relation with response to treatment. To the best of the authors’ knowledge, this is the first case series of Peg IFN-induced lingual hyperpigmentation in patients with hepatitis B, which clinicians should be aware of.
Key Points
1. Tongue hyperpigmentation may be an underreported, but a not so uncommon, adverse effect of Peg IFN therapy.
2. This adverse effect has some cosmetic effect, but subsides when therapy is stopped.
3. Physicians should be aware of this adverse effect, and its reversibility.
INTRODUCTION
Peginterferon α-2b (Peg IFNα-2b), which inhibits viral replication in infected cells, suppresses cell proliferation, induces apoptosis, and exerts an anti-angiogenic effect, is a conventionally used treatment for hepatitis B and C infection. However, with the availability of direct-acting antivirals for hepatitis C, Peg IFNα-2b is used for treating hepatitis B in specific scenarios.1,2 Various dermatological side effects of Peg IFN therapy, including dry skin, alopecia, and exacerbation of autoimmune skin diseases, such as psoriasis and lichen planus, have been reported.3,4 There are also some case reports and series of lingual hyperpigmentation with Peg IFN and ribavirin therapy in patients with hepatitis C5-19 (Table 1). The authors communicate their experience with this rare side effect for the first time, during routine use of Peg IFN in patients with hepatitis B.
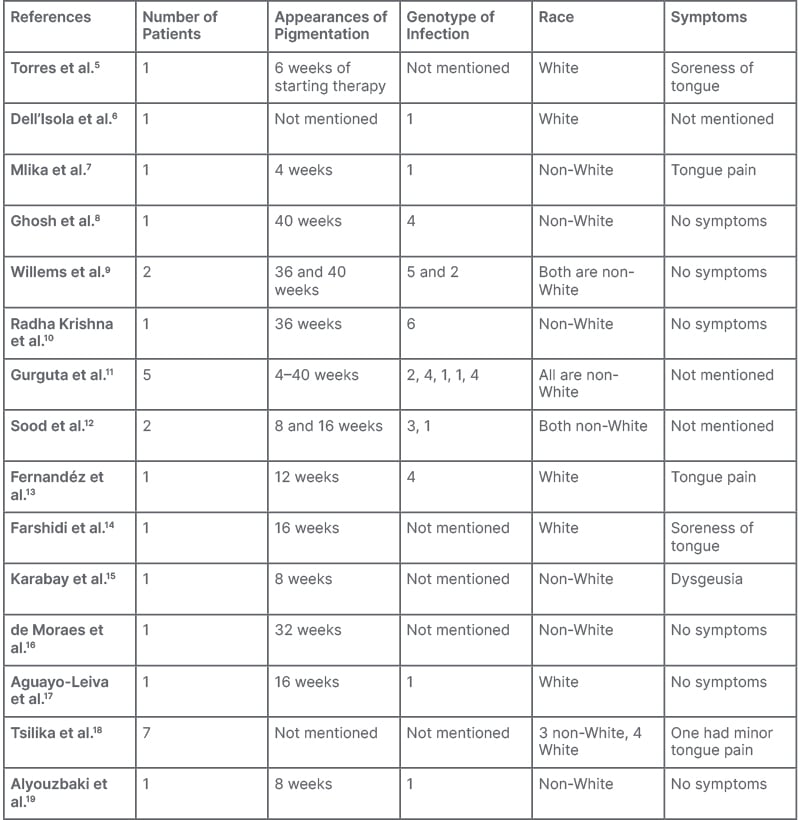
Table 1: Previous documentation.
CASE 1
A 31-year-old female with hepatitis B presented with transaminasemia, e-negative viral load, genotype A and hepatitis B virus DNA of 103 copies/mL, no features suggestive of portal hypertension, and liver stiffness measurement of 7 kPa. The patient was non-hypertensive, non-diabetic, non-alcoholic, and a non-smoker. After initial routine work-up, the authors started her on Peg IFNα-2b 80 μg subcutaneously, weekly. They continued her Peg IFN for 48 weeks, as she met all the criteria of response to therapy. She developed hyperpigmentation at the dorsal and lateral surface of the tongue after 36 weeks of therapy (Figure 1A). She had no other diseases that may cause hyperpigmentation, such as McCune–Albright syndrome, Addison’s disease, nutritional deficiencies, Peutz–Jeghers syndrome, heavy metal exposure, malignant melanoma, neurofibromatosis, etc. She had not received any medication that may lead to hyperpigmentation (e.g., oral contraceptives, bismuth salt, etc). The authors followed the patient at regular intervals off-treatment of Peg-IFN. This hyperpigmentation disappeared gradually after a follow-up period of 36 weeks.
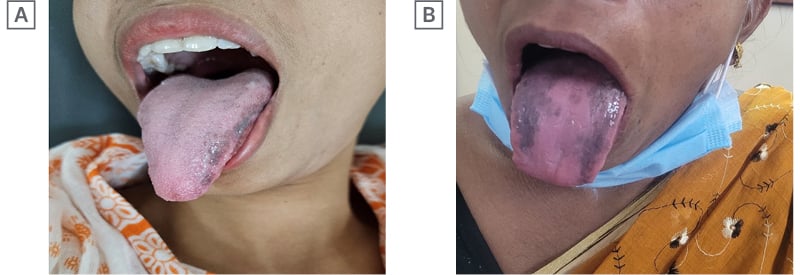
Figure 1: First two documented cases.
CASE 2
A 46-year-old female with incidental detection of chronic hepatitis B with moderate transaminasemia (alanine transaminases [ALT] more than 3 times the norm), genotype A, e-negative disease, viral load of 103 copies/mL, and liver stiffness measurement of 8 kPa, received 48 weeks of Peg IFNα-2b therapy. She had no addiction and no other comorbidities. She too developed tongue hyperpigmentation, albeit on the dorsal surface of the tongue, at 33 weeks of treatment (Figure 1B). The authors regularly followed this patient up to 30 weeks, and the pigmentation faded away. Like in the first case, she had no diseases, and had not received any medication that may cause tongue pigmentation.
CASE 3
A 32-year-old male with chronic hepatitis B, ALT more than five times the norm, e-negative disease, genotype A, viral load of 103 copies/mL, no feature suggestive of portal hypertension, and liver stiffness measurement of 7 kPa, was started on Peg IFNα-2b therapy. He took alcohol occasionally, but had no other addiction or chronic disease. After 46 weeks of therapy, he developed lingual hyperpigmentation at the dorsal surface (Figure 2A). All confounding factor causes of pigmentation were also excluded. Hyperpigmentation gradually vanished after 29 weeks of off-treatment follow-up.
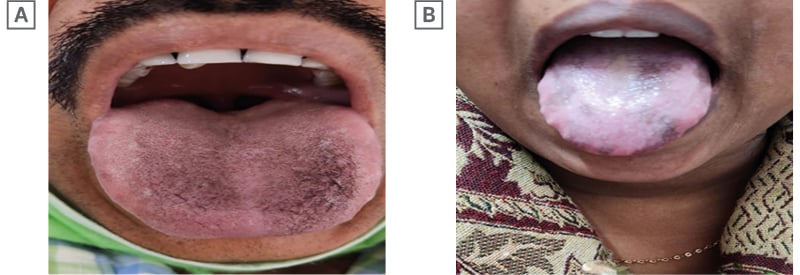
Figure 2: Last two documented cases.
CASE 4
A 37-year-old female with chronic hepatitis B and e-negative disease, genotype A with low viral load (102 copies/mL), transaminases of more than two times the norm, with liver stiffness around 10 kPa, was started on Peg IFNα-2b therapy. The patient was asymptomatic, and had no other chronic disease. She noticed tongue hyperpigmentation around 31 weeks of therapy, which completely disappeared 27 weeks after completion of therapy (Figure 2B). Other factors causing hyperpigmentation were excluded in this patient too.
DISCUSSION
The treatment option for hepatitis B consists of oral antivirals in most cases. In limited scenarios, Peg IFN can be used in patients with low initial viral load (<103/mL), younger age, high ALT, low level of fibrosis in the liver, and genotype A and B.20 Retrospectively, the authors have observed the side effects of routine use of Peg IFN therapy in patients with hepatitis B. They had treated only 21 patients with Peg IFN who met the criteria in the last 5 years. All had e-negative disease and genotype A. Among 21 patients with chronic hepatitis B, the authors came across four cases (19%) of lingual hyperpigmentation. Pigmentation commonly found in tongue can have varied aetiologies, from physiological changes, to oral manifestations of systemic diseases and malignancies.21 The pathophysiology behind this lingual pigmentation is mostly due to deposition of either endogenous (e.g., haemoglobin, haemosiderin, melanin) or exogenous (lead, mercury, bismuth, arsenic, etc.) pigments.21 The presentations can vary from blue/purple vascular lesions, and brown melanotic or haeme-associated lesions, to grey/black pigmentations.21 Considering the temporal association with Peg IFN therapy and appearances, disappearances of lesions, and exclusion of other causes, confirmed the side effect. As past publications were reviewed, to the best of the authors’ knowledge, 28 cases of tongue hyperpigmentation were anecdotally reported, including one by the author of this article, with IFN therapy in patients with hepatitis C.5-19 However, all authors considered it a rare side effect of IFN therapy. These reports suggest female (65%) preponderance, and that populations with dark skin (66.6%) were predisposed.19 To the authors’ knowledge, they are the first to report their experiences in hepatitis B.
In the authors’ series, where they treated a non-White Indian population infected with chronic hepatitis B, three of the cases of tongue hyperpigmentation (75%) were females, and all were dark skinned. As they searched the literature, they noted Willems et al.9 documented that dark-skinned patients receiving interferon therapy showed higher plasma levels of α-melanocyte-stimulating hormone.9 One hypothetic mechanism of these deposits may be that receptors of this hormone up regulation at melanocytes because of interferon, which ultimately increases the melanin production, and causes tongue hyperpigmentation. One study did the biopsy of the tongue in a White patient co-infected with hepatis C and HIV, treated with Peg IFN. They showed an increased number of melanocytes in the basal layer of the epithelium.5 Where normal keratinocyte and melanocyte ratio is 10:1, it was 5:1 in that particular patient. Biopsy was not done in any of the cases, as they were asymptomatic, and the authors had previous experience of this benign self-limiting side effect in hepatitis C.8 Gurguta et al.11 described five cases of similar tongue hyperpigmentation with chronic hepatitis C therapy. All of them were non-White, and infected with various genotypes of hepatitis C infections. Appearance of pigmentation occurred among them between 4–40 weeks of therapy, as in the authors’ cases.
The authors only had patients of genotype A, simply due to selection bias of choosing patients for Peg IFNα-2b treatment. Nevertheless, the same findings theoretically should hold true for all genotypes of hepatitis B. The duration between the start of the treatment and the appearance of the pigment spots ranges from 4–40 weeks, as in the authors’ cases.19 Surprisingly, the sites of involvement were varied, which is a subject of study of interest. As previously described, no dysgeusia or tongue pain, including soreness and burning, were reported by the patients. Gradual improvement of the lesions has been noted in all cases with discontinuation of treatment, which denotes it is a self-limiting side effect. Additionally, the authors need to mention that, although previous reports considered the skin condition of lingual hyperpigmentation a rare side effect, their experience suggests that a good number of patients may have the side effect, which gastroenterologists have to be aware of. As it is a cosmetic effect, this may add to the psychological stress of Peg IFN therapy, particularly in young females.
CONCLUSION
Therefore, the authors can come to a conclusion that tongue hyperpigmentation is an important adverse effect of IFN treatment, irrespective of viral aetiology, that can add psychological stress for patients treated with Peg IFN. Further observation with a higher number of patients on Peg IFN therapy may highlight the exact frequency of this underreported, but possibly not uncommon, side effect.







