Abstract
Non-alcoholic fatty liver disease presents a number of ethical dilemmas. These relate to the potential harms of diagnosing the disease in health, diagnosing a condition for which there is no effective treatment, and variability in specialists’ attitudes to discussing and managing obesity. Erroneous homogenisation of a patient group that is extremely varied in terms of risk factors such as ethnic background, socioeconomic status, and genetic predisposition may result in inappropriate uniformity of approach when counselling patients as to underlying causes. This article will explore these challenges from the perspective of the gastroenterologist or hepatologist who must navigate them. Each section starts with questions posed by patients or comments made by doctors. Caution is suggested before widespread population-based screening is established, and the need for good adherence to referral algorithms is emphasised. Physicians are urged to engage with the condition’s hidden complexities and reflect on their own communication strategies.
Key Points
1. Ethical dilemmas in the management of non-alcoholic fatty liver disease (NAFLD) stem from the potential harms of diagnosing the disease in health, diagnosing a condition for which there is no effective treatment, and variability in specialists’ attitudes to discussing and managing obesity.2.The patient group affected by NAFLD are diverse, in terms of risk factors such as ethnic background, socioeconomic status, and genetic predisposition, so communication strategies should be tailored to each patient.
3. Without straightforward management options, NAFLD screening, referral, and investigation should be carefully considered and outlined at the health service level.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD), the most common hepatological condition in the Western world, is associated with late diagnosis in those who progress to cirrhosis; yet there is no consensus on how to treat it when diagnosed at an early stage.1-4 NAFLD is the hepatological manifestation of metabolic syndrome and is associated with obesity, Type 2 diabetes, and dyslipidaemia; therefore, its diagnosis also has implications for long-term cardiovascular mortality and morbidity. Self-management of obesity, which often drives NAFLD, frequently fails.5 It is a common scenario for a patient to be seen in clinic after having an ultrasound that shows steatosis, and for them to be told about the risk of progression to advanced fibrosis, but for no effective management plan to be offered other than long-term monitoring (the authors’ experience and personal communications). In cases where advanced fibrosis or cirrhosis is identified, surveillance for hepatocellular carcinoma or other complications is considered, but these do not change the patient’s trajectory. In cases of early or moderate fibrosis, the importance of addressing the underlying problem of obesity (if present) can be explored and strategies for losing weight discussed. Referral to bariatric services may be undertaken in selected cases; however, access to surgical treatment is limited and waiting lists are long. The only other avenue is entry into a research study. Many studies involve novel pharmacological agents, are placebo controlled, and last for >2 years; therefore, a large proportion of patients will receive either no or limited treatment (although the placebo effect itself has been shown to reduce alanine aminotransferase by around 10 U/L in trials).6 Though NAFLD is increasingly diagnosed, unified global strategies for management and treatment options are sparse.7 Going forward, tackling the rising epidemic of NAFLD requires a multidisciplinary approach, with hepatologists working closely with primary care physicians, cardiologists, and diabetologists.
For such a common condition, and one with few troublesome early symptoms, NAFLD presents a surprising number of ethical dilemmas, largely derived from our failure to adequately treat the majority of cases.8 This article explores these challenges from the perspective of the gastroenterologist or hepatologist who must navigate them.
“I ONLY HAD THE BLOOD TEST BECAUSE OF A MEDICATION I TAKE; I DIDN’T THINK THERE WAS ANYTHING WRONG WITH MY LIVER…”: DIAGNOSING DISEASE IN HEALTH
Liver disease is largely silent until symptoms associated with decompensation occur. Although there is evidence to suggest many patients with NAFLD go undiagnosed, there is a danger in failing to discriminate between the liver that contains fat (NAFL) and the liver in which fat may be mediating a degree of permanent harm (NAFLD or non-alcohol related steatohepatitis [NASH]).9-11 Historically, it has been accepted that NAFL is largely benign, but there is emerging data to suggest that there may be progression of fibrosis.12 It is difficult to predict this progression as it is dependent on numerous factors such as genetics and environment.13 Age, presence of diabetes, and BMI also play significant roles.14 Despite this reservation, diagnosis of definite disease in all patients with fat in their livers, without reference to strict criteria, may lead to overdiagnosis and its associated harms.15
For example, patients who are given the label of NAFLD may encounter problems when seeking life insurance; others may come away with the impression they are heading towards liver failure (patient communications). A recent meta-analysis shows patients with NAFLD have a high prevalence of depression, and discussions around diagnosis need to be nuanced in order to prevent unnecessary anxiety.16 Studies in another liver condition, hepatitis C infection, have shown that awareness of viraemia negatively impacts quality of life over decades, even when hepatic- or virus-related symptoms are not the cause.17 The reasons for this were unclear, but were felt to relate to anxiety due to the diagnosis, alcohol use, social deprivation, having been homeless at any stage, older age, and methadone treatment.
If, following diagnosis, there are no well-developed guidelines or algorithms to rationalise the pathway, patients may continue to attend specialist clinics for monitoring of liver function, thus increasing the burden on hospital services. The continued referral of patients with simple steatosis to specialist services is costly and may detract from the management of patients who are at higher risk of liver fibrosis and require more intensive monitoring. In order to avoid this, strict referral criteria are required. These have been developed and are based on the exclusion of patients who appear to be at low-risk, based on non-invasive markers such as Fibrosis-4 (FIB-4) or Enhanced Liver Fibrosis (ELF) tests; nevertheless, these are not yet universally embedded.18,19
The danger of overdiagnosis may be increased if population-based screening is adopted. Health systems have different incentives and reimbursement arrangements for the management of conditions that are detected during health screens, and this has to be considered when offering screening tests to whole populations. Several initiatives using community-based FibroScans® (Echosens, Waltham, Massachusetts, USA) have been developed and, although these may benefit the minority who have advanced fibrosis, the advice given to attendees who are found to have liver steatosis must be clear in order to avoid undue anxiety.20 An economic evaluation of a screening programme in Nottingham, UK, using a Markov model, was predicated on the likelihood that pioglitazone, when widely used, would reduce disease progression and morbidity.21 This drug is not in wide use, and the evidence for its efficacy is weak (see below). Moreover, a large observational study of patients with Type 2 diabetes taking this drug found signals towards bladder malignancy and osteoporosis.22 The paper does not explore potential harms due to false positive diagnosis. Increasing access to mobile FibroScan technology has allowed informal ‘roadshow’ screenings, where patients receive an immediate assessment of fatty infiltration and fibrosis. Hepatologists may be in two minds about this. On the one hand, they increase the awareness of silent liver disease; however, on the other, they risk previously healthy men and women walking away with a diagnosis but no clearly defined forward plan other than advice to adopt a healthy lifestyle and visit their GP.
“SO IT’S JUST A BIT OF FAT IN THE LIVER, DOCTOR?”: THAT DOESN’T SOUND TOO BAD!
Patients may leave the consultation thinking NAFLD is not significant unless there is inflammation and subsequent fibrosis. This is a simplistic view as NAFLD is the hepatological manifestation of metabolic syndrome and is associated with obesity, Type 2 diabetes, and dyslipidaemia, with implications for long-term cardiovascular mortality and morbidity. There has been a recent international expert consensus statement to change the terminology from NAFLD to metabolic-associated fatty liver disease.23 Unless patients understand this, efforts to make meaningful lifestyle changes to modify risks may not be successful. Recent meta-analyses have shown that patients with NAFLD have significantly high risks for cardiovascular events,24,25 which is the main cause of mortality in these patients, whereas mortality due to liver events only accounts for a third of the causes.26
In addition to associations with metabolic syndrome, other extrahepatic manifestations include chronic kidney disease, polycystic ovary disease, malignancies, obstructive sleep apnoea, osteoporosis, depression, and cognitive impairment.27,28 There is indeed new evidence to suggest the association between NAFLD and Type 2 diabetes is bidirectional, and that NAFLD could be a precursor of diabetes.29 The theory is that hepatokines such as fetuin-B impair metabolic control, leading to diabetes.30 These considerations raise an important question: should NAFLD be managed by hepatologists alone, or does it need a multidisciplinary clinic?
“THAT DOESN’T SOUND VERY NICE, DOCTOR”: ARE INVASIVE INVESTIGATIONS LIKE LIVER BIOPSY JUSTIFIED?
Although liver biopsy is the ‘gold standard’ investigation to differentiate NAFL from NASH, its role has been controversial, especially in the absence of specific treatment options. It is a useful tool when there is diagnostic uncertainty regarding concomitant liver pathology, borderline non-invasive markers, or to permit inclusion in clinical trials. However, many patients having a liver biopsy for inclusion in clinical trials may not have significant fibrosis and may have undergone an unnecessary invasive procedure. In the context of metabolic-associated fatty liver disease, when there is definitive evidence of metabolic associations of fatty liver disease, is a liver biopsy justified for diagnosis unless the purpose is inclusion in clinical trials? Furthermore, biopsies taken for research and clinical trials raise ethical questions about voluntary consent, and patients misunderstanding that there is a requirement to undergo a biopsy for an intervention.
Though guidelines from various societies like the European Association for the Study of the Liver (EASL), American Association for the Study of Liver Diseases (AASLD), and Asian Pacific Association for the Study of the Liver (APASL) differ slightly in their recommendations for liver biopsy in NAFLD. The general consensus is that it is reserved in cases with uncertain diagnosis or to confirm advanced liver fibrosis.31,32 Liver biopsy is associated with risks such as bleeding, infection, and pain. A recent meta-analysis of liver biopsies reported an overall risk of bleeding of around 2%.33 Patients report significant anxiety associated with biopsy, and studies show that this is associated with higher reported pain.34 Clear discussion about indications, risks, and procedure can help patients make an informed decision and alleviate anxiety.
“SO, WHAT CAN YOU DO ABOUT IT?”: DIAGNOSING DISEASE WHEN THERE IS NO TREATMENT
Patients diagnosed with NAFLD, NASH, and fibrosis will ask what can be done. The reasonable expectation, as in other areas of medicine, is that some form of treatment will be prescribed. In NAFLD, although many agents have been trialled, and some are included in guidelines, there is no highly effective pharmacological intervention. A meta-analysis of 77 trials including 6,287 participants concluded that: “Due to the very low quality evidence, we are very uncertain about the effectiveness of pharmacological treatments for people with NAFLD, including those with steatohepatitis.”35 Vitamin E and pioglitazone feature on many guidelines, but their use in secondary care is not routine. Indeed, vitamin E (with other antioxidants) has been associated with increased overall mortality.36 A recent trial of the farnesoid X receptor obeticholic acid found a high incidence of side effects among patients who took the dose required to reverse fibrosis;37 the U.S. Food & Drug Administration (FDA) did not approve the drug. Research into other agents that interrupt the pathway to fibrosis continue; however, the underlying problem, obesity, appears to be the area where interventions will be most fruitful. Weight loss of 5–10% is associated with improvements in liver function and histological features of NASH, and weight loss following bariatric interventions have shown great promise.38-41 However, non-surgical weight loss is often difficult to achieve, with many patients unable to adhere to diets, while musculoskeletal problems related to previous injuries, or as a result of excess body weight, can restrict options for exercise (personal communications). Surgery, while effective, is implicitly riskier in the short-term, with longer-term complications that are being recognised as decades pass since techniques were refined.42,43 Waiting times for bariatric surgery for those who are selected may be ≥2 years. Less invasive bariatric procedures such as endoscopic sleeve gastroplasty or duodenal ablation appear to be promising alternatives, but are not yet widely available. NAFLD, arguably more than any other condition, represents a paradox. Its prevalence is hugely disproportionate to the available effective management options. This means that many patients will leave the clinic unsure about what to do.
“MAYBE HE WAS BEING CRUEL TO BE KIND, BUT I FELT FAT SHAMED…”: ATTITUDES TO OBESITY AMONG PHYSICIANS
The fact that obesity is stigmatised is well established. In studies dating back over a decade, patients with obesity may have been assumed to be “lazy, unmotivated, lacking in self-discipline, less competent, noncompliant, and sloppy.”44 Discrimination of people with obesity is as prevalent as discrimination based on race or gender.45 How then, does the average physician view patients with obesity and liver disease?
Physicians’ attitudes to obesity vary greatly, and this can have measurable effects on patient outcome in terms of weight loss;46 for physicians do judge and often do harbour negative attitudes.46,47 Even professionals who specialise in obesity have been found to show “very strong weight bias, indicating pervasive and powerful stigma.”48 Ringel and Ditto49 showed that moralisation (the assumption that obesity reflects weakness or diminished responsibility for one’s own body) by physicians was associated with presumptions that patients should be able to control the condition, and with stronger opinions about the possible harms.
Empathy for patients with obesity is not elicited automatically, but can vary according to their perceived success in self-management.50 If simplistic attitudes prevail, and these are communicated to patients (albeit unconsciously), the therapeutic relationship is likely to deteriorate. Hearing that weight gain is a simple mathematical imbalance between calories in and calories out is unlikely to engage a patient constructively. It is very unlikely that patients attending clinic with significant liver disease related to obesity will not have understood the importance of body weight on their lives previously, and the vast majority will have tried to address this, albeit ineffectively. Patients in clinic who request assistance with weight loss are not necessarily shifting the onus of responsibility onto their physician. In one survey, only 20% of patients felt that their doctor should actively contribute to their weight loss management.51 However, general gastroenterologists and hepatologists are not dietitians or psychological therapists, and their skills in counselling patients on how to address their weight are unlikely to be well developed. Large studies of primary care physicians have shown low levels of confidence in their ability to manage this condition, and this could reasonably be extrapolated to doctors working in secondary care.52
Peckham53 observed: “Obesity is becoming increasingly stigmatised as ‘scientific’ health information is incorporated into a pre-existing set of cultural beliefs that fat people are either gluttonous or slothful (or both), and that their lack of self-control and moral fibre is costing millions of pounds each year in medical treatment and lost earnings.”
Encouragingly, Budd et al.,54 in their review of 15 studies on physician attitudes, found that they may have improved between 1990 and 2007. Conversely, studies have shown that the theme of control is important to patients, and that this can be increased or renewed following bariatric surgery.55 The moral complexity of performing surgery on ‘healthy organs’, purely to treat a condition that is secondary to ‘lack of self-control’ opens up a legion of difficult questions regarding choice, utility, and resource allocation.56 Outside of this review of adult medicine, but worthy of comment, bariatric surgery performed on children with obesity highlights the moral dilemmas even more clearly. Children do not have independent medical capacity, but the intervention may well be lifesaving.57
Hepatologists, perhaps more than other medical specialists, see several conditions that are ostensibly related to lifestyle. These include alcoholic liver disease and viral hepatitis acquired through intravenous drug use. There is bound to be variability related to physicians’ personal attitudes, backgrounds, and education, as has been described in relation to people with alcohol or drug dependence.58 Moral responsibility, deservingness of medical attention, and de-prioritisation for scarce resources have been studied extensively in relation to alcoholism and liver failure; it would not be surprising if judgmental attitudes crossed over into NAFLD.59 One way of approaching this tendency is to reflect on the fact that many patients were pushed onto the path of obesity, metabolic syndrome, and liver disease long before they had responsibility for their own health.
“I’VE BEEN OVERWEIGHT FOR AS LONG AS I CAN REMEMBER…”: HEREDITARY AND SOCIAL DETERMINANTS OF DISEASE
Clinically significant NAFLD is associated with numerous genetic, hereditary, ethnic, and social determinants, over which patients have no control. It has parallels with alcoholic liver in this regard.60 At the genetic level, polymorphisms in PNPLA3, TM6SF2, and MBOAT7 have major impacts in both; this is unsurprising, as the mechanism of both diseases involves lipid dysregulation.60,61 In a study correlating liver biopsies to genetic status, fibrosis was associated with MBOAT7 and PNPLA3 polymorphisms.62 Recent evidence has suggested that gut microbiota dysbiosis may also predispose to liver damage.63 While it is hoped that identifying such underlying factors could allow us to tailor management and surveillance, for physicians facing patients in the present, these associations may serve to remind them that the scarred liver is not just a manifestation of weak will.64
More complex still, and less well understood, are the influences of race and wealth. The influence of deprivation is felt at a young age, as shown in studies of paediatric populations with confirmed liver disease on MRI or biopsy.65 Alarmingly, socioeconomic status may extend its influence to the post-transplant period, one study showing that graft survival was negatively affected among children from poorer areas.66 Underlying risks driving racial and socioeconomic disparities in obesity prevalence may be poor education, unemployment, greater access to poor quality foods, poor access for physical activity, targeted marketing of unhealthy foods, and poor access to healthcare or referrals.67 In the UK, Sir Michael Marmot’s68 report ‘Fair Society Healthy Lives’ clearly showed that obesity prevalence correlates to socioeconomic quintile. Ethnicity and NAFLD and its complications are clearly linked; it is unclear how much of this is due to genetic profiles and dietary changes resulting from urbanisation. From high to low, incidence varies across the Middle East, South America, Asia, North America, Europe, and Africa; globally, it affects approximately 25% of the population.69
“I’LL REFER YOU, BUT I CAN’T GUARANTEE THEY’LL PUT YOU ON THE LIST…”: TRANSPLANTATION IN OLDER PATIENTS WITH COMORBIDITY
NASH cirrhosis is diagnosed later in life than other forms of cirrhosis, and patients are more likely to have other cardiovascular comorbidities.70,71 When hepatocellular carcinoma is found, it tends to be at a later stage.72,73 Transplanted patients are older (typically over 65), and early complications are more common.74,75 One-year survival is lower compared to other indications, according to one report.76 The patient with end-stage liver disease from NASH, therefore, presents a management challenge as movement onto the liver transplant waiting list may be impeded by concerns about perioperative risk and graft utility. Referring for transplantation is therefore a complex decision. In the authors’ experience, patients are often declined.
Then, there is the issue of disease recurrence. Unlike alcohol-related liver disease or viral hepatitis, where lifestyle or medical treatment are assured to reduce the risk of de novo disease in the graft, NAFLD is likely to return. A meta-analysis showed that the incidence of recurrent NAFLD was 82% at 5 years.76 Cirrhosis related to recurrent NASH was 11–14%. An expert group that convened to discuss the phenomenon post-transplant fatty change agreed that NASH in this context was more aggressive but that, thus far, evidence was lacking to show that graft failure is more common, or overall patient survival is impaired.77 These concerns have not led to reduced rates of transplantation for NASH on the basis of reduced utility. However, much thought is being given to strategies to reduce disease recurrence. Potential post-liver transplant treatments (beyond lifestyle and diet) include liraglutide, a glucagon-like peptide 1 receptor antagonist, and bariatric surgery.78,79
From the standpoint of the general hepatologist, it is clear that considerable thought needs to be given before referring patients for transplantation, and that false hope should not be given to the older patient with overt, or a high chance of covert, comorbidity.
The ethical issues and challenges associated with NAFLD and potential solutions are presented in Table 1.
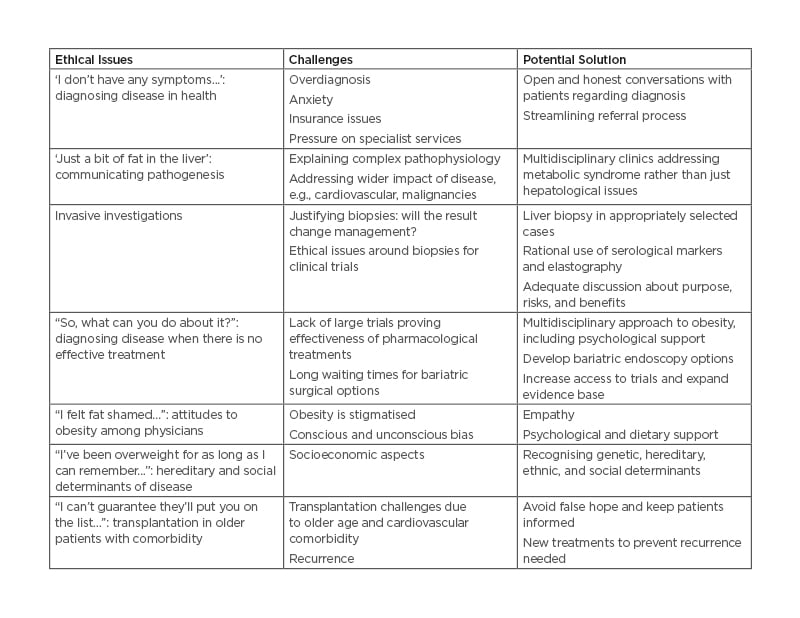
Table 1: Ethical issues and challenges associated with non-alcoholic fatty liver disease and potential solutions.
CONCLUSION
Management of NAFLD is not as straightforward as it first looks. There is no virus to suppress, no single behaviour to modify, no easy prescription, and no straightforward route to transplantation. For gastroenterologists and hepatologists who see patients with NAFLD, a good understanding of hereditary factors, significant uncertainties around management, and their own potential biases or presumptions is required. Services should strive to design and embed clear criteria for referral, investigation, and subsequent discharge, if appropriate. More broadly, careful thought should be given to population screening, for there is a danger that healthy people, or those with mild disease who are unlikely to suffer liver-related morbidity, will acquire the label of disease without a clear forward plan.







