INTRODUCTION
Portal hypertension is the major determinant of outcomes in patients with cirrhosis, defined as the presence of a hepatic venous pressure gradient (HVPG) of more than 5 mmHg. Mild portal hypertension is defined as HVPG of 6–10 mmHg. The major determinant of mild portal hypertension is hepatic fibrosis with little systemic contribution.1 On the other hand, clinically significant portal hypertension (CSPH) is defined as an HVPG ≥10 mmHg. The development of CSPH is regarded as an important event in the natural history of patients with cirrhosis. Physiologically, this heralds the onset of hyperdynamic circulation, sympathetic overactivation, splanchnic vasodilatation, and bacterial translocation, making CSPH a systemic condition unlike mild portal hypertension.2 Clinically, this stage is associated with the development of gastro-oesophageal varices, increased risk of decompensation, and mortality due to hepatic decompensation.3 Patients with compensated cirrhosis with CSPH (especially those with established varices) decompensate more frequently than those with mild portal hypertension.4,5 The gold standard for the diagnosis of CSPH is HVPG estimation. Clinically, gastro-oesophageal varices and abdominal collaterals signify CSPH as an HVPG of 10 mmHg or more is required for their formation.6 Liver stiffness measurement (LSM) is a marker of liver fibrosis and inflammatory activity and correlates precisely with HVPG in patients with mild portal hypertension. After the onset of CSPH, this correlation becomes less precise.7 LSM cut-offs using vibration-controlled transient elastography (a bedside tool) with or without platelet count can rule in or rule out CSPH, at least in viral, alcohol-related, and non-obese metabolic dysfunction-associated steatotic liver disease (MASLD).8 Baveno VII consensus has laid down risk prediction rules to detect CSPH by non-invasive estimation (Rule of 5; Figure 1).1
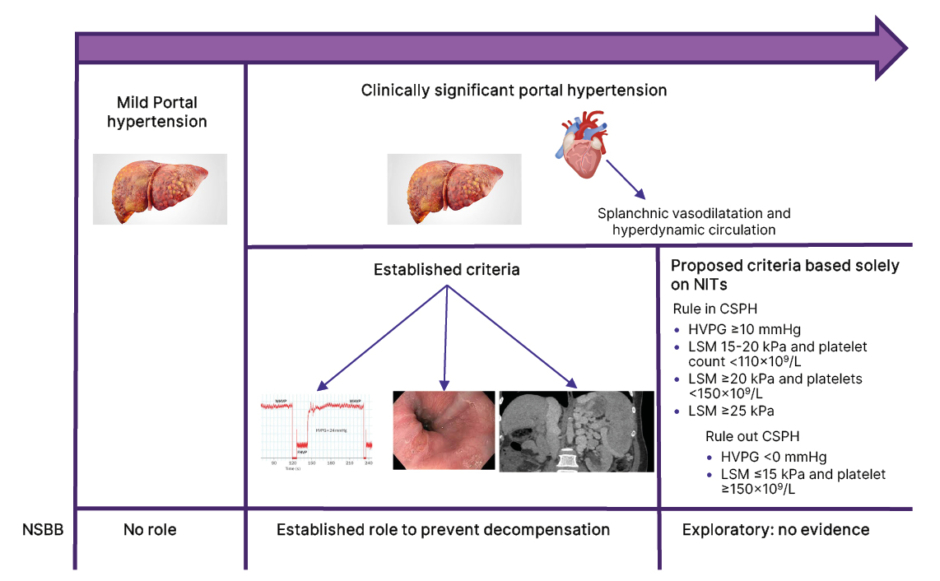
Figure 1: Conceptual flow diagram showing the role of non-selective beta-blockers in patients with different stages of portal hypertension.
CSPH: clinically significant portal hypertension; HVPG: hepatic venous pressure gradient; LSM: liver stiffness measurement; NIT: non-invasive test; NSBB: non-selective beta-blocker.
Splenic stiffness measurement is a marker of portal blood flow and is emerging as a more accurate marker of CSPH than LSM.9 It can be a useful adjunct to improve the accuracy of LSM to detect CSPH, particularly in patients stratified in the grey zone with LSM.10 Novel biomarkers such as von-Willebrand factor and serum metabolomics, when used alone or in combination with Baveno VII risk prediction rules, are also being assessed to detect CSPH non-invasively and predict the risk of hepatic decompensation.11,12
NON-SELECTIVE BETA-BLOCKERS IN CLINICALLY SIGNIFICANT PORTAL HYPERTENSION: ESTABLISHED INDICATIONS
Over four decades ago, an increased understanding of portal circulation physiology laid down the foundation for non-selective beta-blockers (NSBB) in the management of portal hypertension in selected patients.13 NSBBs dampen the hyperdynamic circulation and splanchnic vasodilatation, the two self-perpetuating mechanisms of CSPH. Therefore, their role in the absence of CSPH and the hyperdynamic circulation is not defined.2 The indications for NSBB have been refined over this time. These were originally used for the prevention of variceal bleeding in patients with large varices or red signs. With the recognition of ascites rather than variceal bleeding as the most common decompensating event in the natural history of liver disease, and the fact that 25% of patients with CSPH decompensate with ascites over 2 years, a paradigm shift of using NSBB beyond prophylaxis against variceal bleeding emerged recently. 3,14
Early signs of this shift became apparent 2 decades ago in a randomised placebo-controlled trial of timolol to prevent the formation of varices in compensated cirrhosis.15 In this trial, NSBB did not prevent the onset of varices or reduce the incidence of hepatic decompensation. The trial included all patients with compensated cirrhosis and portal hypertension (HVPG >5 mmHg). Post-hoc analysis of this trial showed that patients with HVPG ≥10 mmHg decompensated more frequently than those with HVPG <10 mmHg over 4 years of follow up (30% versus 10%).16 This trial quantified the concept of CSPH and identified the sub-group with higher rates of decompensation and more likely to benefit from portal pressure-lowering therapies. The critical threshold in stratifying these patients was HVPG ≥10 mmHg. The most reliable surrogate marker of HVPG ≥10 mmHg is the presence of varices irrespective of size and radiological collaterals.1 The shift in the role of NSBB from bleed prophylaxis to prevention of all-cause decompensation became more apparent after the PREDESCI trial. This trial randomised patients with compensated cirrhosis and CSPH based on HVPG estimation to NSBB (carvedilol or propranolol) and placebo for each corresponding NSBB.4 NSBB reduced the incidence of hepatic decompensation compared to placebo (16% versus 27%; hazard ratio [HR]: 0.51; 95% CI: 0.26–0.97; p=0.041) primarily by reducing the incidence of ascites (HR: 0.44; 95% CI: 0.20–0.97; p=0.0297). This pivotal trial laid down the concept of treating CSPH to prevent all-cause decompensation. However, sub-group analysis of this trial showed the net benefit of this strategy to be restricted to those with varices on endoscopy. This was subsequently validated by an individual patient meta-analysis showing the benefits of NSBB in those with compensated cirrhosis with varices irrespective of their size.17 The ongoing UK-based multicentre pragmatic randomised trial conducted in patients with cirrhosis and small varices (BOPPP, ISRCTN10324656) will give more information in this regard.18 This trial, which includes both compensated and decompensated patients, was initially designed to study the role of NSBB for variceal bleed prophylaxis but was later modified to evaluate all-cause decompensation as its primary endpoint. As of now, there is increasing evidence for treating patients with compensated cirrhosis with any varices with NSBB to prevent the first decompensation. This strategy has been endorsed by the Baveno VII consensus and the American Association for the Study of Liver Diseases (AASLD).1,19 The BOPPP trial, with over 700 participants already randomised, will provide further valuable evidence in a contemporary cohort of patients resulting in more generalisable findings.18
NON-SELECTIVE BETA-BLOCKERS IN CLINICALLY SIGNIFICANT PORTAL HYPERTENSION: PROPOSED INDICATIONS
Overall, the evidence for treating CSPH only exists when it is confirmed using HVPG or stratified by the presence of varices. The bulk of discussion and controversy of NSBB in patients with compensated cirrhosis is centred around their role in patients who do not have clinical signs of CSPH. As discussed earlier, while HVPG is helpful in this condition, its invasive nature limits its application in clinical practice. While rates of decompensation in this sub-group (HVPG ≥10, no varices) are lower than in those with established varices, it is still around 25–30% in 2 years, especially in aetiologies like alcohol-related liver disease with ongoing consumption.16
Patients with compensated cirrhosis and non-invasive surrogates of CSPH but without varices represent a unique sub-group as there is a lack of evidence of NSBB to improve outcomes in this state. While recent data suggests that non-invasive risk prediction rules predict distinct decompensation, there is no data to support their utility alone in starting NSBB in the absence of varices (Table 1).20-25
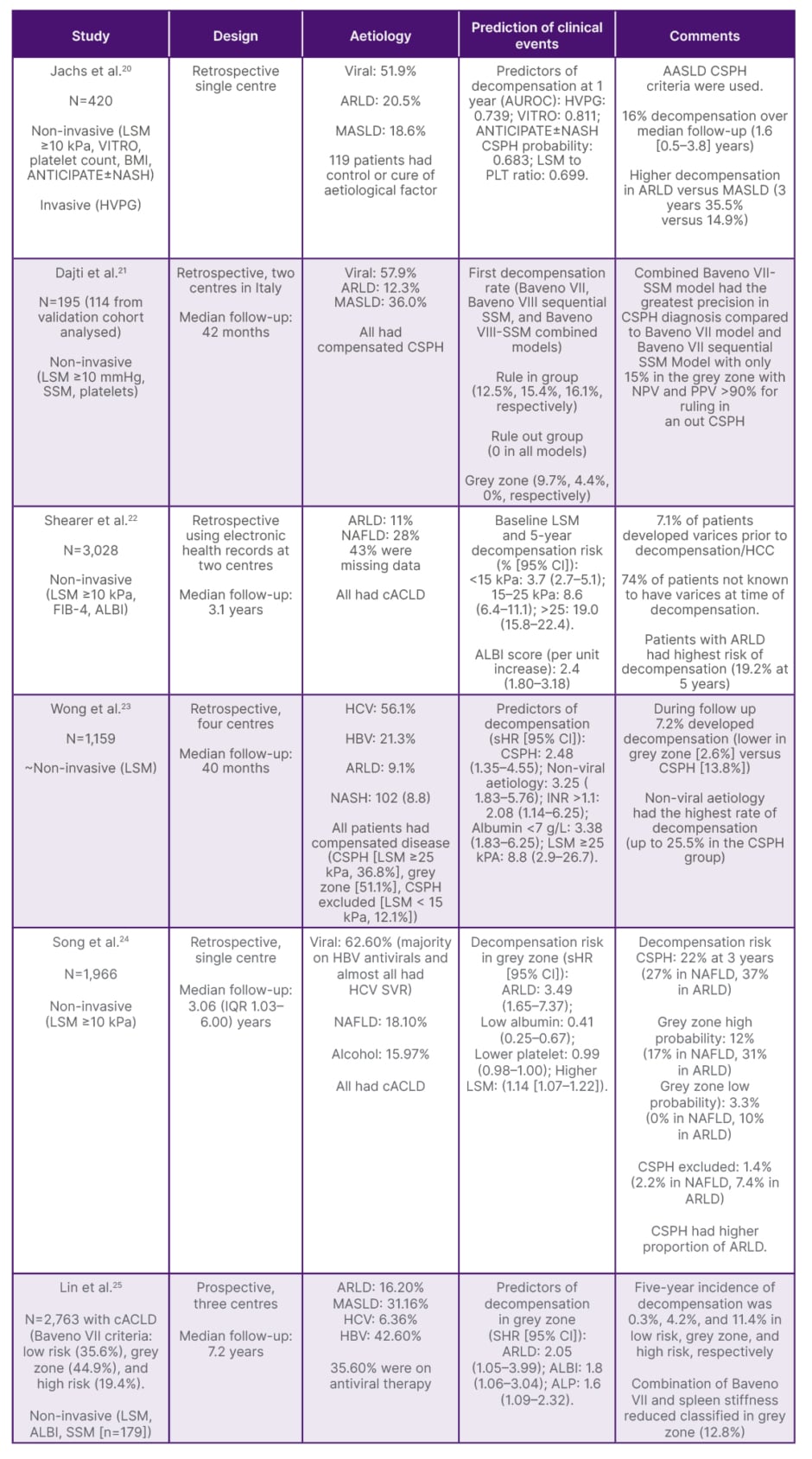
Table 1: Key studies based on non-invasive criteria to predict the risk of decompensation.
ALBI: albumin-bilirubin; ARLD: alcohol-related liver disease; AUROC: area under the receiver operating characteristic curve; cACLD: compensated advanced chronic liver disease; CSPH: clinically significant portal hypertension; FIB-4: fibrosis index based on four factors; HBV: hepatitis B; HCV: hepatitis C; HVPG: hepatic venous pressure gradient; IQR: interquartile range; LSM: liver stiffness measurement; MASLD: metabolic dysfunction-associated steatotic liver disease; NAFLD: non-alcoholic fatty liver disease; NASH: nonalcoholic steatohepatitis; NPV: net present value; PLT: platelet count; SSM: spleen stiffness measurement; SVR: sustained virologic response; VITRO: von Willebrand Factor antigen to platelet ratio.
The concept of starting NSBB in patients with compensated cirrhosis with only non-invasive criteria of CSPH (i.e., without varices/HVPG estimation) is extrapolated from the PREDESCI trial.4 However, there are significant drawbacks to this proposed extrapolation. First, the efficacy of NSBB was significantly lower in patients with CSPH but without varices in the PRESDESCI trial. The benefits of NSBB were restricted to only patients with varices, which constituted 56% of the trial population and hence impacted the overall study results. Unlike HVPG, LSM did not have the same discriminatory ability to predict decompensation in this trial. Second, the overall event rate in this trial was low, with only a moderate effect size. Third, the predominant aetiology of liver disease in the PREDESCI trial was hepatitis C virus.
Since the advent of direct-acting antivirals, treatment of hepatitis C has drastically changed. There is emerging data that shows that NSBB may not be needed in patients with compensated viral cirrhosis who achieve virological suppression/cure as the rate of decompensation is significantly reduced.26,27 The predominant aetiology of liver disease in current pragmatic trials of NSBB in CSPH is alcohol (unpublished data from BOPPP and CALIBRE) and MASLD, which have higher rates of decompensation.18,28 Patients with MASLD can even decompensate before the HVPG threshold of 10 mmHg.29 Fourth, the positive predictive value of non-invasive criteria depends upon the baseline prevalence of CSPH and is likely to be different in different cohorts.30 This has resulted in inconsistent demonstration of the benefit of these non-invasive criteria in validation studies for CSPH.31,32 Due to differences in effect size, different aetiology of liver disease, and more importantly, a different method of CSPH estimation (HVPG versus LSM estimation), extrapolation of evidence of treating CSPH from PREDESCI cannot be applied to patients only satisfying non-invasive diagnosis of CSPH without concomitant HVPG estimation or demonstration of varices. To change clinical practice the evidence for this non-invasive test-based strategy must be generated.
DOWNSIDE OF NON-SELECTIVE BETA-BLOCKERS FOR ALL
NSBB have numerous side effects. Around 10% of patients have contraindications to NSBB, and 15% of patients discontinue NSBB due to adverse effects.33 In the timolol study, the incidence of serious adverse effects in NSBB arm was 18% compared to 6% with placebo.15 Data on the impact of NSBB on quality of life is not known. With an uncertain benefit of NSBB in those with non-invasive CSPH estimation and listed side effects, unknown impact on quality of life, and indefinite treatment, the practice of NSBB for all is an oversimplified extrapolation of evidence with a concerning risk of lowering net benefit of these drugs (with more patients being treated with this strategy who do not decompensate irrespective of being on NSBB).
SUMMARY
In summary, we have limited drugs to treat portal hypertension, and NSBB are among the most important agents when used in highly selected patients. There is now an established role for NSBB to prevent decompensation in patients with CSPH detected either on HVPG estimation or by the presence of varices. However, there is no evidence to initiate NSBB to reduce decompensation based on non-invasive estimates of CSPH exclusively in the absence of varices and HVPG <10 mmHg/unavailable HVPG. In the absence of prospective data from randomised trials, the strategy of NSBB for all based only on non-invasive risk tools should be strongly discouraged as NSBB are likely to lose their net benefit with intervening adverse effects if their prolonged use is expanded without evidence (Figure 1).