Abstract
The relatively indolent nature of well-differentiated neuroendocrine tumours (NET) and their proclivity to be hormonally active warrants aggressive multimodal treatment, even for advanced stage disease. Good results have been reported in well-selected patients with a median of 23 liver metastases (LM) from NET treated with surgical resection combined with intraoperative radiofrequency ablation. We report the case of a patient who underwent percutaneous laser thermal ablation (LTA) of 22 small LM from NET, treated over three consecutive sessions. After 2 years, five new LM were detected and treated with LTA. At present, 82 months after the first LTA session, the patient is still alive and disease-free. Due to enabling the use of one to four optical fibres at once to tailor the thermal lesion size to the nodule size, LTA could represent the ablation technique of choice in the presence of multiple, small, and variably sized LM.
INTRODUCTION
Liver metastases (LM) from neuroendocrine tumours (NET) occur with variable frequency depending on the primary disease, ranging from 5–10% for carcinoid tumours to 75% for glucagonoma.1 The relatively indolent nature of well-differentiated NET and their proclivity to be hormonally active warrants aggressive multimodal treatment even for advanced stage disease. Indeed, aggressive cytoreduction using combined treatments, such as surgery, transarterial chemoembolisation, and thermal ablation, can improve both survival and quality of life, and, to date, many guidelines for the management of NET recommend the removal of at least 90% of LM whenever possible.2-4 With this aim, surgical resection is considered the gold standard treatment, but only 10–20% of patients with NET are suitable candidates for resection. Ablative therapies can be used in place of, or as an adjunct to, resection. In brief, they can preferentially be used for deep small lesions (diameter <4 cm) and recurrent lesions and can be considered as an adjunct to resection in patients with extensive bilobar disease. Thermal ablative techniques can deliver thermal energy, either cooling (cryoablation) or heating the tissue. Radiofrequency ablation (RFA), microwave ablation, and laser thermal ablation (LTA) raise the temperature of the tissue to between 60°C and 100°C, producing coagulative necrosis. LTA, according to the technique proposed by Pacella et al.5 and modified by Di Costanzo et al.,6 uses 300 µm bare optical fibres introduced into the tumour through 21 gauge needles. The ability to place one to four fibres at once using a multisource device makes LTA the most flexible ablation technique.7,8 A bare-tip fibre provides an almost spherical thermal lesion of 12–15 mm in diameter, and the lesion size can be increased up to 4–5 cm by using multiple fibres with the pull-back technique.5,6 In our opinion, such novel features make LTA the ablation technique of choice when treating multiple liver tumours of varying size. This paper reports the case of a patient who underwent LTA of 22 small LM from NET.
CASE REPORT
In November 2007, a 26-year-old woman underwent complete pancreatic resection for insulin-secreting NET. The tumour was well differentiated with a Ki-67 expression level of 3% and a chromogranin A level of 92 ng/mL. Contrast-enhanced computed tomography (CECT) showed multiple (≥20) LM, ranging from 5–14 mm in size. In January 2008, the patient began medical therapy with somatostatin analogues (octreotide followed by pasireotide); in January 2010 she underwent transarterial chemoembolisation. In January 2011, restaging CECT showed stable disease.
Given the low proliferation index of the tumour and the indolent course of the disease, ultrasound (US)-guided percutaneous LTA was planned to remove as many LM as possible. LTA was preferred to other ablation techniques because of the high number of LM and their small size to balance the need of obtaining a good safety margin with the need to spare normal liver parenchyma. Medical therapy with pasireotide was not discontinued and LTA was performed on an inpatient basis using a commercially available system composed of an US device and a laser unit (Echolaser, Elesta Srl, Florence, Italy). The laser source was a semiconductor diode with a wavelength of 1,064 nm, and a multisource device enabled the use of up to four fibres at once (Figure 1). After local anaesthesia with 10 mL 1% lignocaine and conscious sedation with intravenous midazolam and remifentanil, the laser fibres were introduced into the tumour through 21 gauge needles under US guidance.
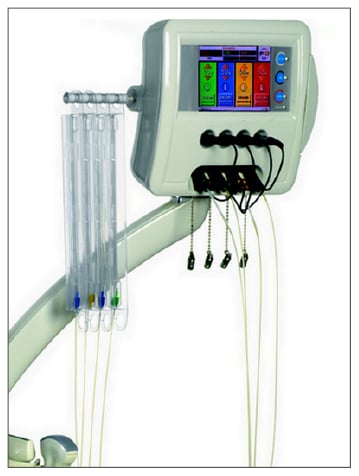
Figure 1: Laser ablation machine with multisource device with four bare optical fibres connected.
The laser machine was set at a power of 5 W, and 1,800 J per fibre were delivered in 6 minutes. One, two, or three laser fibres were used for nodules ≤7 mm, 7–12 mm, and 12–14 mm in diameter, respectively; the pull-back technique was used when the anteroposterior diameter of LM exceeded 10 mm.
In total, 22 LM were ablated over three sessions. Primary technical success and 1-month technique efficacy, defined according to the standardisation of terminology and reporting criteria for image-guided tumour ablation,9 were 100%. In brief, technical success addressed whether the tumours were treated according to the protocol and were covered completely by the ablation zone and was assessed by contrast-enhanced US (CEUS) performed immediately after the end of each treatment session (Figure 2 and 3); 1-month technique efficacy referred to complete ablation of the tumours and was assessed by CECT or CEUS performed 1 month after the end of the third planned session of LTA. The patient was then followed up every 3 months with alternating CECT and CEUS and remained free from disease until October 2013, when five new LM ranging from 5–12 mm were detected. A further LTA session was performed with primary technical success and 1-month technique efficacy of 100%. At the time of writing (30th November 2017) the patient is still alive and disease-free, tumoural hormonal secretion is normalised, and she is being supplemented with insulin because of the previous complete pancreatectomy.
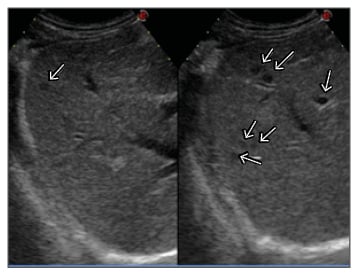
Figure 2: CEUS scan of the right liver lobe before the first session of LTA showing seven small hypoechoic liver metastases (arrows).
CEUS: contrast-enhanced ultrasound; LTA: laser thermal ablation.
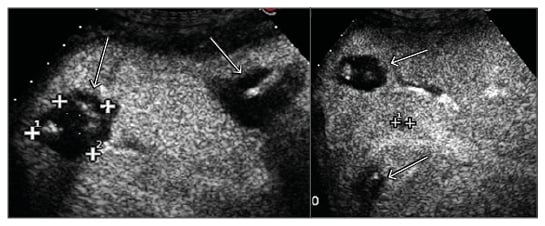
Figure 3: CEUS scan of the right liver lobe after the first session of LTA showing four ablation areas completely covering six metastases. One metastasis was still viable and was treated in the following LTA session (cross-shaped markers).
CEUS: contrast-enhanced ultrasound; LTA: laser thermal ablation.
DISCUSSION
Systemic medical therapy is marginally effective in NET, and the reported 5-year survival of patients with LM from NET treated medically ranges from 0–30%.10 In contrast, aggressive cytoreduction using combined multimodal treatments has been reported to achieve 5-year survival rates ranging from 48–83%.1,2 Surgical resection is considered the aggressive approach of choice, but it is only recommended when at least 90% of the tumour burden can be removed, and only 10–20% of patients with NET are suitable candidates for resection because the disease is too extensive.11 Moreover, the 5 and 10-year recurrence rates after resection are 84% and 94%, respectively, with a median time to recurrence of 21 months.1 Consequently, the need to frequently retreat patients makes less invasive methods of aggressive cytoreduction a quite interesting option, either as a primary therapy or in a multimodal approach, or as an adjunct to hepatic resection.12 Image-guided thermal ablation offers the possibility of a minimally invasive technique that is usually associated with less morbidity than resection, decreases tumour volume, preserves most of the normal liver, and can be repeated several times. Among the thermal techniques that can destroy neoplastic nodules by heating the tissue, LTA offers some advantages in clinical settings. The diameter of the needles is considerably smaller than RFA electrodes or microwave ablation antennas, making LTA safer and more suitable for ablating tumours in at-risk locations or in locations that are difficult to reach.6-8 Moreover, the use of a multisource device that allows the placement of one to four fibres at once enables tailoring of the thermal lesion size to the nodule size, which allows an acceptable safety margin in tumours ranging from 5–6 mm to 3–4 cm in diameter, contemporaneously sparing the normal parenchyma as far as possible.
LM from NET are often multiple, small, variable in size, and very slow growing. Therefore, it is possible to treat patients with indolent disease with numerous and small (≤3 cm) LM, even with multiple treatment sessions over a period of years.13 In our department, LTA, associated or not with other cytoreductive treatments, such as surgical resection and transarterial chemoembolisation, has become the ablation technique of choice in this subtype of selected patients because of its unique qualities.14
Some authors obtained good results adopting a very aggressive approach to LM from NET. In particular, Elias et al.15 reported a 3-year survival rate of 84% in 16 patients with a median of 23 LM per patient who were treated with surgical resection combined with intraoperative RFA. In four cases this was preceded by preoperative selective portal vein embolisation; however, approximately 60% of the LM was surgically excised, and 40% underwent intraoperative RFA. In our patient, a higher number of LM were successfully treated using only percutaneous US-guided thermal ablation. Twenty-two LM were ablated over three sessions in 2011, and a further five LM were ablated in 2013; at present, the patient is still alive and disease free 82 months after the first ablation session and 128 months after the diagnosis of pancreatic insulin-secreting NET with multiple LM.
A single case is little more than an anecdote and does not allow any conclusions to be drawn, but this case report suggests that a very aggressive approach can yield very effective results in well-selected patients. Many physicians think that aggressive cytoreduction should be reserved for patients presenting with only a few LM,13,16 but in our opinion the indication for multimodal treatment, including surgery, transarterial chemoembolisation, and thermal ablation, should be expanded in the subgroup of patients exhibiting a low natural tumour burden slope and good general status. In these patients, when thermal ablation is planned to treat a high number of LM, maximum sparing of normal parenchyma is critical, and ablation must be as limited as possible in volume and optimally adapted to the size of the LM to reduce the risk of liver failure. To this aim, due to it enabling the use of one to four fibres according to the size of the LM, in our opinion LTA should be considered the ablation technique of choice.








