Abstract
Patients with autoimmune liver disease frequently fit diagnostic criteria for more than one condition. Up to 12.5% of autoimmune hepatitis (AIH) and primary sclerosing cholangitis (PSC) cohorts have a label of AIH/PSC overlap. There can be an interval of many years between the diagnoses of the two conditions, and the sequence in which they are made is unpredictable. Issues exist with the use of diagnostic criteria validated for AIH in patients with AIH/PSC overlap. There are no agreed criteria for the diagnosis of AIH/PSC overlap, it is based on a combination of biochemistry, autoantibody profile, cholangiogram, and liver histology. A positive diagnosis of AIH/PSC overlap impacts therapeutic options and prognosis. There is a beneficial role for immunosuppression, albeit with a higher relapse rate and evidence of progressive liver disease despite immunosuppression in some cases. Liver related outcomes sit somewhere between the constituent diseases, with better outcomes than PSC but poorer outcomes than AIH. There is an increasing body of data for patients with AIH/PSC overlap undergoing liver transplantation for end-stage disease.
Nearly half of patients with autoantibody positive liver disease in childhood have autoimmune sclerosing cholangitis (ASC). ASC patients are differentiated from those with AIH by having abnormal cholangiograms. Histological analysis shows chronic hepatitis in <50% of ASC cases. The biochemical response to immunosuppression in ASC patients is less than that seen in AIH patients, and cholangiograms commonly show progressive disease. Transplant-free survival of the ASC population is poorer than in AIH.
INTRODUCTION
The autoimmune liver diseases (AILD) have been categorised classically as either autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), or primary biliary cholangitis (PBC). The AILD are a heterogeneous group of conditions with differing pathogenesis, patterns of hepatic injury, and clinical outcomes.1 Despite this, AIH, PSC, and PBC are frequently grouped together as the AILD because of similarities in clinical presentation, immunological markers, and treatment options.
A subgroup of patients with AILD share common features relating to the different subtypes of AILD; these have been termed the ‘overlap syndromes’ or ‘variant syndromes.’ The significance of these overlap syndromes in AILD remains controversial.2,3 Do patients simultaneously have two diseases? Are they the product of inaccurate diagnostic criteria that results in the diagnosis of two conditions when patients only have one? Is there, in fact, a continuum between the two disorders? (Figure 1)
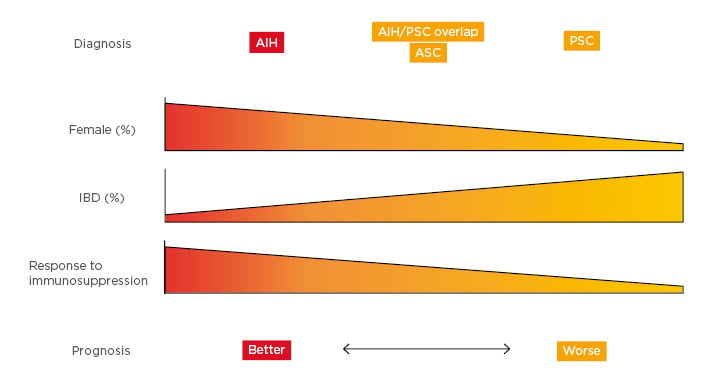
Figure 1: A number of demographics and clinical characteristics differ between autoimmune liver diseases, indicating a better or worse prognosis.
There is a spectrum between AIH and PSC with overlap found in the middle.
AIH: autoimmune hepatitis; ASC: autoimmune sclerosing cholangitis; IBD: inflammatory bowel disease; PSC: primary sclerosing cholangitis.
This review will summarise the overlap syndromes that share features of AIH and PSC, namely AIH/PSC overlap and autoimmune sclerosing cholangitis (ASC). The authors will review the different presentations between children and adults, which are important as patients transition to adult services.
ADULTS
Demographics
Adults diagnosed with AIH/PSC overlap are significantly younger at the time of diagnosis than those with AIH (AIH/PSC diagnosis age: 24–27 years, compared to 39–46 years for PSC patients).4-6 Analysis of the United Network for Organ Sharing (UNOS) database of patients who underwent liver transplantation for PSC categorised by age group, identified a higher prevalence of AIH/PSC in the 18–39-year-old age group (2.1%) than in older patients (1.0% of patients aged 40–59 and 0.5% in those aged >60 years).7 The proportion of adult males with AIH/PSC overlap is 69–81%, which is higher than seen in AIH, which is more prevalent in females.5,6,8
Frequency
The frequency of AIH/PSC overlap varies based on the diagnostic criteria used9 and whether the prevalence has been taken as a proportion of AIH or PSC patients. Biliary changes have been identified in 24% of patients with AIH on liver histology10 and magnetic resonance cholangiopancreatography (MRCP);11 however, these cases were not related to a cholestatic syndrome and were thought to be secondary to fibrosis. The diagnosis of AIH/PSC within cohorts of AIH patients ranges from 1.7–12.5%.4,6,11,12 Larger studies of PSC patients have a definite diagnosis of AIH/PSC in 1.4–9.0%, with up to a further 33.0% with probable AIH/PSC.13-15
Inflammatory Bowel Disease
The rates of concurrent inflammatory bowel disease (IBD) diagnosis in patients with AIH/PSC varies significantly between series (ranges between 13–89%) but appears to be less prevalent than in PSC.4,15-17 Cholangiography was performed to further investigate a subgroup of patients with AIH who were undergoing annual surveillance endoscopy and diagnosed with IBD.18 At least 29% of those with AIH and IBD had features of PSC which were previously unrecognised. If a patient with AIH develops IBD then further evaluation for PSC is likely warranted.
Timing of Diagnoses
In patients who presented with AIH, the subsequent diagnosis of AIH/PSC overlap was made at a mean interval of 5–9 years later, with cases of up to 15 years from presentation.16,17 When PSC was the initial diagnosis the mean interval to AIH/PSC overlap was 3.3 years.15 No cases of small duct PSC progressing to AIH/PSC overlap have been reported.19 AIH appears to be the more common primary diagnosis (in 31–63% of cases) compared with PSC (19–44% of cases), and in a significant proportion of patients the diagnosis is contemporaneous (19–42%).8,15,19 Despite the interval between presentation and a formal diagnosis of AIH/PSC often being a number of years, closer analysis of the initial histology frequently identifies features of both disease processes at presentation.8 A diagnosis of AIH/PSC overlap should be considered at all stages in a clinical course, but particularly early in the diagnosis and if atypical features develop.
Diagnosis
AIH/PSC overlap is mainly seen in young adults, in whom there is a characteristic clinical, biochemical, immunological, and histological picture of AIH with a classical cholangiogram of PSC.20,21 Up to 94% of AIH/PSC overlap patients have antinuclear antibodies, antismooth muscle antibodies, or anti-liver-kidney antibodies at titres of ≥1:40, which is comparable to AIH and higher than in PSC.14,22 Aspartate aminotransferase (AST) level at presentation in AIH/PSC is lower than in AIH,12 while serum globulins and IgG levels are higher in AIH/PSC than in PSC.14 However, there is a significant challenge in making a definitive diagnosis of AIH/PSC because the diagnostic scoring systems used may have only been validated in AIH and there is no dedicated diagnostic criteria for AIH/PSC overlap.22,23
There have been three iterations of the International Autoimmune Hepatitis Group (IAIHG) scoring system for AIH (published in 1993, 1999, and 2008).24-26 All three IAIHG scoring systems are dedicated to AIH and only 2 of 250 patients in the 2008 derivation cohort had AIH/PSC overlap. Although there are similarities between the IAIHG scores there are also key differences. These may reflect the changes in prevalence of AIH/PSC reported in studies that have used different scores. The original 1993 IAIHG scoring system deducts points for elevated alkaline phosphatase (ALP) to aminotransferase ratio and for biliary features on histology.24 Out of a group of 114 patients with PSC who were scored using the 1993 IAIHG score, 2% were found to have definite AIH and a further 33% were probable for AIH.13 This is likely to be a high false positive rate, reflecting the similarities between patients with AIH and PSC and inaccuracies of the scoring system, as opposed to the true prevalence of AIH/PSC. Some modifications were made to the 1999 IAIHG scoring system, and 28 of the 40 patients with PSC who were rescored were reclassified from probable AIH to not AIH.25 In a larger series of 113 PSC patients who had their 1993 and 1999 IAIHG scores calculated, the 1999 score was a lower numerical score.15 Further analysis of 89 patients with AIH using both scores reclassified 15% of cases, a demotion using the 1999 IAIHG score was mainly related to the presence of biochemical and serological characteristics of biliary disease.27 The score was simplified in 2008, with removal of the liver enzyme profile, response to therapy, and deductions for biliary features on histology.26 The 2008 IAIHG score has excellent specificity for PSC: among 147 PSC patients 0.0% were definite and 1.4% probable for AIH.28
The performance of these scoring systems in AIH/PSC overlap have only been reported in a few studies involving small patient numbers. In nine patients with AIH/PSC overlap there was no difference in the 1993 and 1999 scores, with eight definite AIH and one probable AIH in both.15 The 1999 score was applied to three patients with AIH/PSC; of the three patients, one was graded as probable AIH and two were not AIH.29 The 16 patients with AIH/PSC had a lower 1999 score compared to those of AIH patients.5 The 2008 score was probable or definite AIH in 65% of 17 patients with AIH/PSC,22 although this was 0% when the score was applied to an additional cohort of three patients.30
When assigning a diagnosis of AIH/PSC, experts advise caution in using the IAIHG scoring system in clinical practice and advocate the importance of clinical judgement.31 When a diagnosis of AIH/PSC overlap is suspected, key diagnostic tools are MRCP and liver histology, and a final decision can be made as a composite of these results irrespective of IAIHG score (Figure 2).
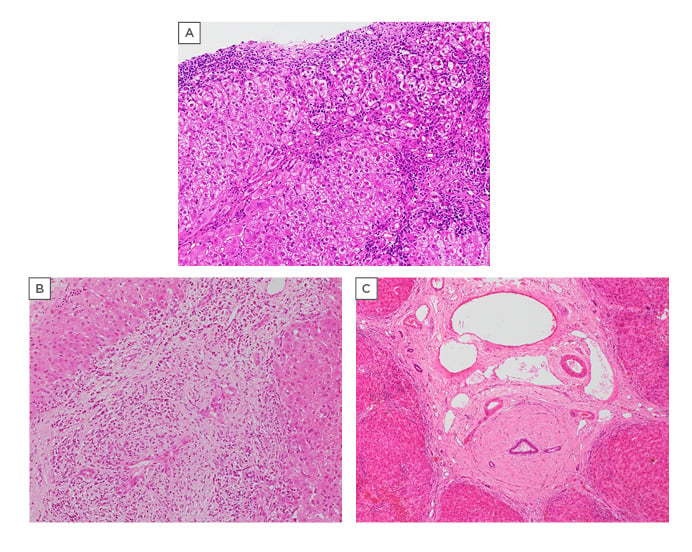
Figure 2: Sequential liver histology from a patient with an initial diagnosis of autoimmune hepatitis and subsequently autoimmune/primary sclerosing cholangitis overlap.
The patient was a 14-year-old girl who presented with ALT 320 UI/L, raised IgG, and positive autoantibodies (ANA 1/160 and ASM 1/160).
A) Autoimmune liver disease with features of autoimmune hepatitis only. Liver biopsy at age 14 years. Sections show advanced-stage chronic hepatitis, with marked lymphoplasmacytic portoseptal inflammation with plasma cell enrichment and moderate interface activity. Hepatocyte rosettes and emperipolesis activity were also observed at higher magnification. Bile duct lesions were not identified (H/E, 100X).
B) Autoimmune liver disease with chronic cholangiopathy. Liver biopsy of the same patient at age 19 years, with radiological features of a cholangiopathy. Obvious bile duct lesions and persistent interface activity, lymphoplasmacytic. The main indication at this time was a cholangiopathy (H/E, 100X).
C) End-stage autoimmune liver disease with sclerosing cholangitis features. Explant of the same patient at age 21 years, with radiological features of an established cholangiopathy. Diffuse periductal concentric fibrosis (sclerosing cholangitis type lesions) was observed. The hepatic parenchyma shows end-stage cirrhosis (H/E, 40X).
AIH: autoimmune hepatitis; ALT: alanine aminotransferase; ANA: antinuclear antibody; ASM: antismooth muscle antibody; H/E: haematoxylin and eosin; PSC: primary sclerosing cholangitis.
Caution should be used with regards to the biliary changes that can be seen in AIH, which do not represent a cholangiopathy of PSC.10,11
Treatment
Treatment in AIH/PSC overlap is a significant challenge due to the small number of studies and lack of randomised controlled trials. Therapeutic options are extrapolated from the management of the constituent syndromes.2
Data on the benefits of immunosuppression in AIH/PSC overlap are variable. There is some data suggesting a benefit from corticosteroids in PSC patients with histological features of AIH, higher bilirubin and alanine aminotransferase (ALT),32 and in patients with AIH/PSC and large duct cholangiopathy.19 Normalisation of ALT was achieved in 88% of patients with AIH/PSC overlap; however, this took a mean of 26 months, much longer than for AIH.8 Remission is achieved less frequently with immunosuppression in AIH/PSC than in classical AIH (22% versus 64%;21 73% [when combined with AIH/PBC] versus 95%).22 Univariate analysis of overlap syndromes (AIH/PSC and AIH/PBC analysed together) detected overlap syndromes were associated with a suboptimal response to immunosuppression.6 Relapse upon reduction of immunosuppression (combination of corticosteroids and azathioprine) was seen in 44% of patients with AIH/PSC overlap; however, the patients responded to increased levels of immunosuppression.8 There is a particularly poor response to immunosuppression in those with small duct AIH/PSC.19 Response to immunosuppression in AIH patients with histological biliary changes is not suggestive of PSC; however, they have similar outcomes to those without.10
In small case series of patients with initially immunosuppression-responsive AIH and subsequent diagnosis of PSC, there is biochemical relapse upon reduction of immunosuppression or progressive cholestasis.17,33 These suggest that in some cases the cholangiopathy has developed despite adequate immunosuppression and may not respond to immunosuppression.
A group of four patients with AIH/PSC overlap, who were either azathioprine non-responders or azathioprine intolerant, were treated with mycophenolate mofetil as a second-line steroid-sparing agent.34 There was a biochemical response in all patients, and three of the four patients achieved remission. Other second-line immunosuppressive agents that have been used in AIH, such as tacrolimus, have not been reported in AIH/PSC overlap.
Combination therapy using immunosuppression (maintenance 10–15 mg per day prednisolone and 50–75 mg per day azathioprine) and 15–20 mg/kg per day ursodeoxycholic acid (UDCA) was associated with a reduction in AST over 5 years in seven patients with AIH/PSC overlap.35 However, there was no change in their cholestatic enzymes (ALP and gamma glutamyl transferase [GGT]). Among the 16 patients with AIH/PSC overlap, a reduction in ALT within 6 months was seen with immunosuppression, irrespective of combination with UDCA.8 These studies raise the question as to whether UDCA provides an additional benefit to immunosuppression in AIH/PSC overlap. The effect of UDCA monotherapy was assessed in seven patients, only 29% achieved remission or a good response in aminotransferase reduction.19
Given the paucity of evidence in the area, guidelines currently recommend empirical combination therapy with immunosuppression and UDCA in AIH/PSC overlap, and liver transplantation in end-stage disease.36 On a practical level, the care of patients should be individualised to address their own balance of hepatitis and cholestasis. Immunosuppression should be targeted at the hepatic component and UDCA at the cholestatic component. The response to therapy can be assessed through a combination of clinical, biochemical, histological, and radiological parameters. As evidenced from the literature, the phenotype of patients can switch during the course of their disease, and modifications in therapy should be made accordingly (Figure 3).
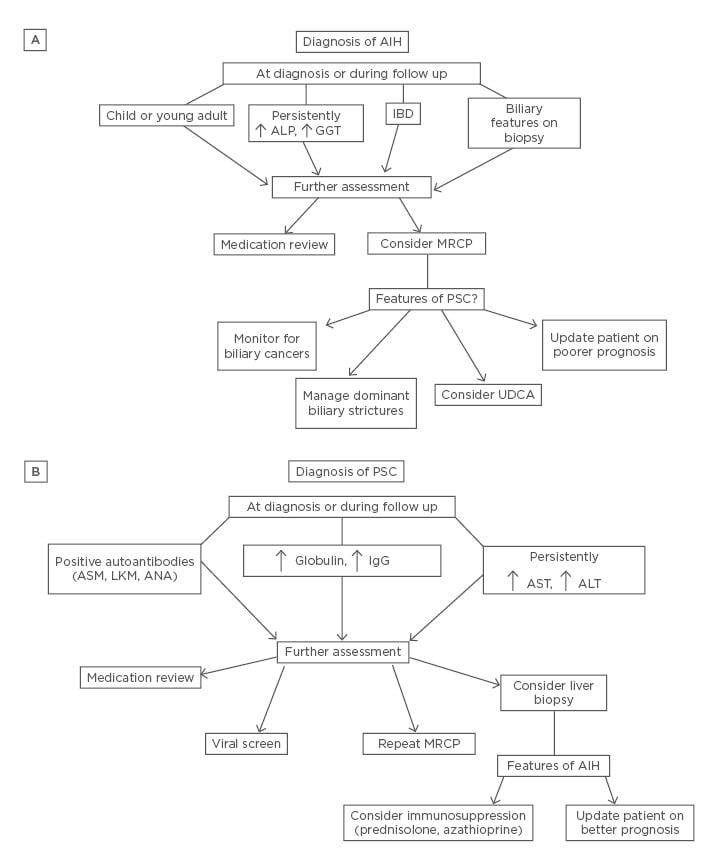
Figure 3: A schematic for the proposed management, either at first diagnosis or during follow up, of patients with AIH (A) or PSC (B) or with features of AIH/PSC overlap.
AIH: autoimmune hepatitis; ALP: alkaline phosphatase; ALT: alanine aminotransferase; ANA: antinuclear antibody; ASM: antismooth muscle antibody; AST: aspartate aminotransferase; GGT: gamma-glutamyltransferase; IBD: inflammatory bowel disease; LKM: liver-kidney microsomal antibody; MRCP: magnetic resonance cholangiopancreatography; PSC: primary sclerosing cholangitis; UDCA: ursodeoxycholic acid.
Outcomes
Despite treatment with currently recommended therapies, progression is common (Figure 2). Over a mean of 12 years follow-up in patients with AIH/PSC overlap on treatment, the proportion with cirrhosis increased from 19% at presentation to 56% at the end of observation.8 There is a wide range (14–80%) reported in the literature for the requirement of liver transplantation for end-stage AIH/PSC overlap and it is limited to small case series with variable duration of follow up.5,16,35,37,38
Long-term patient survival is poorer in AIH/PSC compared with AIH (odds ratio: 2.08; p=0.039),5 and liver-related death and liver transplantation is more common (33% versus 8%; p=0.05).21 The outcomes from AIH/PSC overlap are better than PSC, with no increase in Mayo score prognostic risk index during follow up, no cases of cholangiocarcinoma (15% in PSC), and no deaths (26% in PSC).35
Following liver transplantation, patients with overlap AILD (AIH/PSC and AIH/PBC) had a higher rate of recurrence of disease in their transplanted liver at 5 years compared to single AILD (overlap 53%, AIH 16%, and PSC 18%), but comparable graft and patient survival.39 Recurrent disease was diagnosed in two patients with AIH/PSC overlap, one of whom had features of AIH/PSC overlap and the other had only those of PSC.
PAEDIATRIC AND YOUNG ADULTS
From early reports of children with PSC it was clear that there were many clinical similarities between PSC and AIH, and in fact these patients had often initially been managed as having AIH.40,41 In approximately a third of these PSC cases, the diagnosis was only made after subsequent investigations, including cholangiography, which revealed features of PSC.
A study that systematically evaluated consecutive children with liver disease and positive antibodies consistent with AIH, with screening cholangiogram and liver biopsy, found ~50% had abnormal cholangiograms.42 The term ASC was used for those with abnormal cholangiograms and positive autoantibodies, who had different characteristics to those with normal cholangiograms. The condition has only been described in paediatric populations.
Demographics
The age at diagnosis in paediatric AILD does not appear to differ between subtypes. No difference was seen between AIH/PSC overlap and PSC (11.3 versus 11.5 years),43 or ASC and AIH (11.8 versus 10.5 years).42 However, PSC exhibits a slight male predominance compared with AIH/PSC overlap, with 64% of males presenting with PSC compared to 55% with AIH/PSC overlap.43 A sex difference was not seen between AIH and ASC subgroups (79% versus 55% female).42
Frequency
A diagnosis of AIH/PSC is much more common in paediatric populations than in adult cohorts; in a large multicentre cohort of 781 children with PSC, 33% had AIH/PSC overlap.43 When consecutive children who presented to a single centre with suspected AILD and positive autoantibodies underwent liver biopsy and cholangiogram, a diagnosis of ASC was made in 49% and the remainder were diagnosed with AIH.42
Inflammatory Bowel Disease
Coexistent IBD is present in 63% of patients with AIH/PSC overlap, which is fewer than the 82% seen in PSC (p<0.001).43 ASC patients, on the other hand, more frequently had IBD than AIH (44% versus 18%; p=0.03), and had fewer cases of autoimmune disease in first degree relatives (37% versus 71%).42
Diagnosis
The diagnostic criteria used for AIH/PSC overlap in children is similar to that used in adults. It involves an abnormal cholangiogram or liver histological features of PSC, in combination with a probable or definite classification on simplified AIH criteria.43 The biochemical profile in AIH/PSC overlap was of higher AST and higher ALT, with comparable ALP and GGT to PSC. They also had higher globulin fraction and IgG, and more were antinuclear antibody positive (62%) and antismooth muscle antibody positive (61%).
Although lacking formal diagnostic criteria, ASC was first described in patients with suspected AILD, a positive autoantibody test, and an abnormal cholangiogram.42 This differs from a diagnosis of AIH/PSC overlap because the only feature of AIH required is a positive autoantibody test. The patients had lower bilirubin levels, lower AST levels, and a lower ALP:AST ratio than AIH. All had positive autoantibodies, and 74% had positive antismooth muscle antibodies. Histological analysis in ASC identified less lobular activity, less portal tract inflammation, lower histological inflammatory activity index, and more acute or chronic cholangitis than in the AIH patients. Only 23% had a histological diagnosis of chronic hepatitis on their index liver biopsy, 42% had sclerosing cholangitis, and 19% chronic hepatitis with biliary features.
Treatment
Most ASC patients (85%) were treated with 2 mg/kg per day prednisolone (maximum dose 60 mg);42 however, of those with abnormal baseline AST, 61% did not normalise, compared to all those with AIH treated with prednisolone. In total, 59% of ASC patients required 1–2 mg/kg per day of azathioprine, either due to increasing AST on tapering prednisolone or prednisolone side effects. Further escalation of therapy to penicillamine, cyclosporin, or colchicine was initiated in 22% of patients due to persistent AST elevation. Subsequent data from the same centre have shown 89% response to second-line mycophenolate mofetil in azathioprine non-responders in AIH, compared to only 25% in ASC.44 Outcomes from specific treatment regimens have not been reported for children with AIH/PSC, although 81% have been treated with UDCA.43
Outcomes
Patients with AIH/PSC overlap have similar outcomes in terms of event-free survival and transplant-free survival to PSC patients.43,45 No cases of cholangiocarcinoma have been reported in the paediatric literature for AIH/PSC and are limited to PSC only.43
Follow up assessment in ASC patients was undertaken with biopsies in 17 patients and endoscopic retrograde cholangiopancreatography in 17 patients.42 Histology revealed a significant decrease in histological inflammatory activity index score from baseline biopsy, which was comparable to the improvement seen in the AIH patients. However, the cholangiograms showed progressive intra and extra-hepatic cholangiopathy in 47% of patients. This may, in part, explain the significantly poorer transplant-free survival for ASC compared to AIH, with 15% of the ASC patients requiring liver transplantation during the study period.42 The 10-year transplant-free survival from other series is 89% in ASC, which is comparable to paediatric PSC patients.46 When PSC, ASC, and AIH are compared, the chances of a complication of liver disease within 5 years of diagnosis are 37% PSC, 25% ASC, and 15% AIH, respectively.47 The limited data from these studies suggest that the outcomes for patients with ASC sits between AIH and PSC.
CONCLUSION
There is limited literature on AIH/PSC overlap and ASC. Not only is the number of studies small, but the data is heterogeneous, in part related to the lack of consensus on diagnostic criteria and the variety of scoring systems used across different studies. Despite this, there appears to be sufficient evidence that patients with AIH/PSC overlap and ASC have a different clinical course and different response to therapy than patients with AIH and PSC alone.
In children, and possibly young adults, with AIH, it is reasonable to routinely perform a MRCP at diagnosis to assess for a cholangiopathy. Otherwise an evaluation of the biliary tree should be limited to those with AIH and an incomplete response to immunosuppression or ensuing cholestasis (Figure 3A). Diagnosing a cholangiopathy may allow for therapeutic biliary intervention, heightened awareness of poorer prognosis, lower threshold for investigating for IBD, and cholangiocarcinoma surveillance.
Performing a liver biopsy in patients with PSC to look for evidence of AIH should be restricted to those with a strong autoantibody profile or persistently elevated transaminases (Figure 3B). If an AIH component is detected, then the patient may derive benefit from immunosuppression. However, there should be an awareness of the poor response rates to immunosuppression in these patients, in terms of progressive cholangiopathy and chronic liver disease despite adequate immunosuppression, prior to embarking on therapy.








