Abstract
Splenosis should be suspected when a patient has a history of trauma or abdominal surgery. Intrahepatic splenosis is a rare disease that is often difficult to distinguish from liver malignancy, especially hepatocellular carcinoma. The cause of intrahepatic splenosis may be though the auto-transplantation of splenic tissue on the surface of the liver. The authors report a case of intrahepatic splenosis that presented as a liver tumour in an 81-year-old female treated for autoimmune hepatitis, who had no history of splenectomy or abdominal trauma. Laparoscopic hepatectomy was performed and the specimen demonstrated characteristic histopathological findings of the spleen. Only one case of a patient who had no history of splenectomy or abdominal trauma has been reported in the literature. It may be hypothesised that erythropoiesis induced by local hypoxia in the chronic hepatitis may cause the growth of splenic erythrocytic progenitor cells, which have migrated via portal vein to the liver.
Key Points
1. Generally speaking, intrahepatic splenosis (IHS) has been thought to occur after splenectomy or abdominal trauma. In this case report, IHS occurred in the patient who had no splenectomy or abdominal trauma.2. Here, the authors explain the imaging diagnosis of IHS, and differential diagnosis from hepatic malignancy. The cause of IHS was inferred from previous literature.
3. When an atypical hepatic tumour is found, it is important to keep in mind that IHS could be one of the differential diagnoses, even if the patient has no history of splenectomy or abdominal trauma.
INTRODUCTION
Intrahepatic splenosis (IHS) is thought to be formed by the intrahepatic proliferation of splenic tissue by autograft after splenic injury or splenectomy. It is important to include IHS in the differential diagnosis based on splenic injury and history of abdominal surgery, as it can be difficult to differentiate it from hepatic malignancy, especially hepatocellular carcinoma (HCC), on imaging diagnosis. In this report, the authors describe a case of IHS diagnosed by pathological examination in a patient with no abdominal trauma or history of surgery.
CASE REPORT
An 81-year-old female was being observed by their primary care physician for autoimmune hepatitis. The patient was found to have elevated liver enzymes and was referred to the authors’ internal medicine department on suspicion of HCC. The patient was asymptomatic, and had no history of any abdominal trauma or surgery.
Patient History
In early 2013, the patient was examined for abnormal liver function. Their antinuclear antibody was 1:1280; their IgG level was 3475mg/mL; and their hepatitis virus markers were negative. There was no other cause of hepatitis. As a result, the patient was treated with ursodeoxycholic acid as a patient with autoimmune hepatitis. The patient’s details were taken on admission, including height: 157 cm; weight: 47 kg; clear consciousness; body temperature 36.1 °C; blood pressure 151/87mmHg; pulse 88 /min; well-conditioned; no anaemia or yellowing of the ocular conjunctiva; and a flat, soft abdomen. Laboratory findings on admission showed a mildly elevated aspartate transaminase of 35 IU/l and lactate dehydrogenase of 254 U/l. Both hepatitis B surface antigen and hepatitis C virus qualitive were negative, and tumour markers carcinoembryonic antigen, α-fetoprotein, and protein induced by vitamin K absence-II were within the reference values.
Abdominal contrast CT was performed. An examination in early 2017 showed a 13 mm large low-density area in segment Ⅷ (S8) of the liver, faintly contrasted in the arterial and portal phases, and indistinguishable from normal tissue in the equilibrium phase. The enhancement pattern was different from typical HCC, and more like haemangioma. At this point, the authors diagnosed a haemangioma of the liver, and followed-up 10 months later with a CT examination in early 2018. The mass had increased to 30 mm. In the arterial phase, part of the margin was faintly enhanced, and the margin was predominantly faintly enhanced in the equilibrium phase (Figure 1). The contrast effect was stronger than the previous CT examination.
Abdominal gadoxetic acid-enhanced MRI (Gd-EOB-MRI) findings include a 30 mm large mass in S8, showed as a pale contrast effect on a part of the limbus in the arterial phase and a partially missing image in the late phase. The hepatocellular phase also showed low intensity and diffusion-weighted imaging, which revealed a slightly high signal area (Figure 2). These findings were consistent with HCC. Abdominal ultrasonography: a heterogeneous mass was seen in S8 of the liver. Colour doppler showed no blood flow signal within the mass.
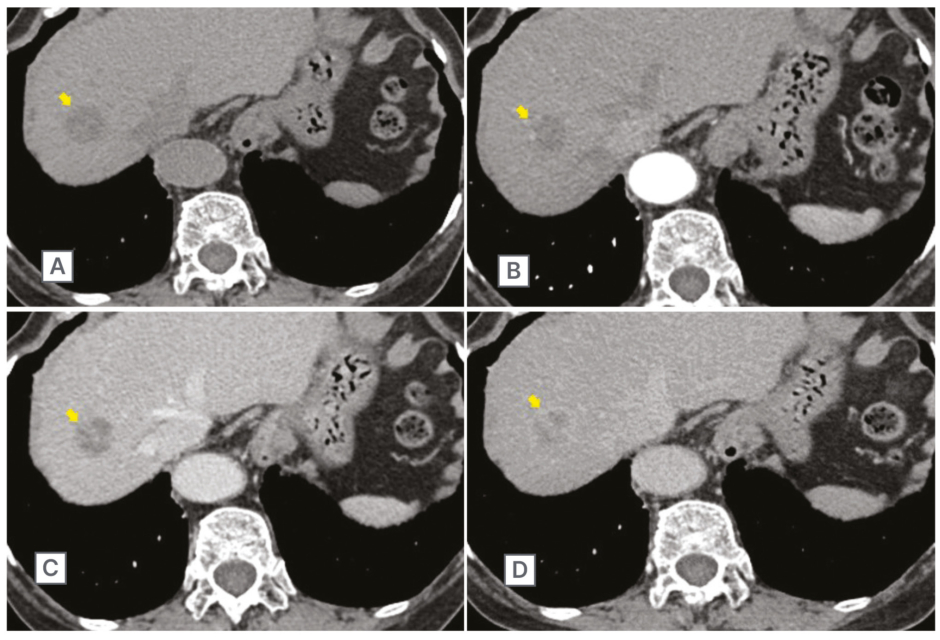
Figure 1: Abdominal contrast CT scan in March 2018.
This scan showed a 30 mm large mass lesion (arrow) in segment Ⅷ of the liver (A). The enhancement effect progressively increased in the arterial phase (B), portal phase (C), and equilibrium phase (D).
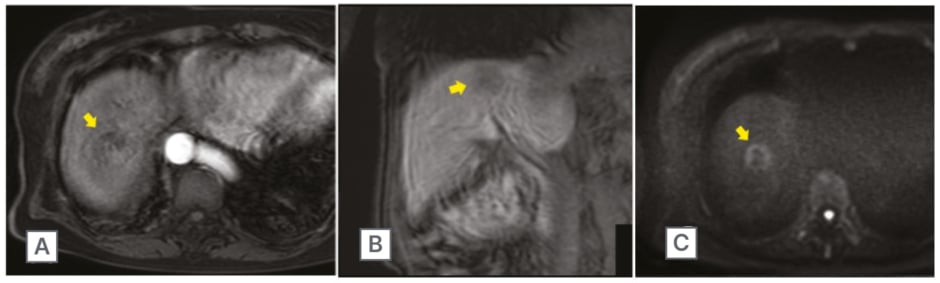
Figure 2: Gadoxetic acid-enhanced MRI scan of the abdomen.
Scan of the abdomen showed a 30 mm large mass lesion (arrow) in segment VIII. In the arterial phase, the lesion had a faint contrast effect at the margins (A), and in the late phase changed low signal (B). Diffusion-weighted imaging revealed slightly high signal area (C).
Treatment and Course
Based on the above examinations, the mass was growing, and HCC could not be ruled out, so the patient underwent laparoscopic partial hepatectomy in spring 2018. The liver surface was uneven due to coarse regenerative nodules, giving the appearance of chronic hepatitis. The surface of the mass in S8 was white, depressed, and trailing. There was no mass in the abdominal cavity.
Pathological Examination
The mass was 20 mm in size, had a capsule, and was reddish brown with white cord-like material (Figure 3A). The surface of the mass was depressed (Figure 3B). Microscopic examination showed a thick connective tissue capsule, some arteriovenous inflow from the liver, one fibrous nodule containing arteriovenous vessels thought to be a splenic column, and an associated lymph follicle (Figure 3C). The histopathological findings confirmed that the lesion was composed of typical splenic tissue (i.e., IHS). In the background liver, mononuclear cells infiltrated around the portal and periportal areas, which was consistent with autoimmune hepatitis (Figure 3D). Post-operative course was uneventful, and the patient was discharged on post-operative Day 8.
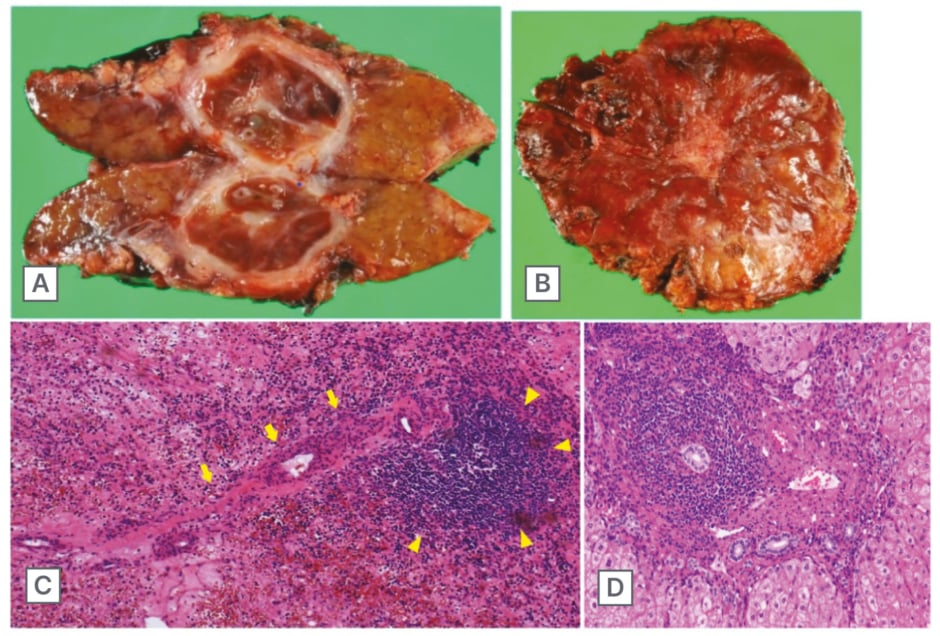
Figure 3: Cut surface of the resected specimen.
Analysis of the cut surface showed an encapsulated, round shape mass and 20 mm in diameter (A). The liver surface was uneven due to coarse regenerative nodules, giving the appearance of chronic hepatitis, and the surface of the mass was depressed (B). Histopathological examination revealed a fine fibrous structure containing large amounts of blood, with a single splenic arteriovenous-like structure (arrow) and associated lymph follicular formation (arrowhead) (C). The background liver revealed dense portal and periportal mononuclear cells infiltrate, forming interface hepatitis that is a relatively typical finding of autoimmune hepatitis (D).
DISCUSSION
Splenosis was first described in 1939, when Buchbinder et al.1 named the splenic tissue that remained viable in the abdominal cavity after splenectomy following traumatic splenic injury as splenosis. It is an ectopic autograft, unlike a congenitally developed accessory spleen, and all patients have a splenic history of trauma or abdominal surgery. Some reports estimate that as many as 26–65% of patients with splenic rupture develop splenosis.2,3 They are often discovered 10–20 years after splenic injury or abdominal surgery, and the number varied from 1–400 in the literature.4 The omentum, mesentery, and serosa of the intestinal tract are common sites for splenosis.5,6 There are some case reports that have occurred in the thoracic cavity.7,8
IHS is very rare, and even with today’s advanced diagnostics there are still cases mimicking as hepatic malignancies.9-13 Toh et al.14 demonstrated a review of 59 cases in their study between 1939 and 2019. IHS should be diagnosed by the triad of history of splenectomy or abdominal trauma, absence of risk factors for liver malignancy, and typical contrast pattern on imaging studies.14,15 (The contrast pattern of CT and MRI is described below.)
The mechanism of IHS is generally thought to be the growth and proliferation of disseminated splenocytes, like other cases of splenosis on the serosa of the organs. What then should be the explanation in this case? IHS without a history of abdominal trauma or surgery has only been reported in one case.16 In that case, chronic hepatitis C infection was present in the background liver. Kwok et al.17 suggested the particular mechanism of IHS. They hypothesised that erythrocytic progenitor cells somehow strayed into the liver via the portal vein after splenic trauma, and ectopic splenomegaly was induced by erythropoiesis due to local hepatic hypoxia.17 In this mechanism, the presence of background viral hepatitis or other liver disorder may be a factor in causing intrahepatic hypoxia.18,19 In a present case, the background liver was histologically severe hepatic degeneration by autoimmune hepatitis. Intrahepatic hypoxia could have occurred, and there was thought to exist a potential of IHS.
In terms of imaging diagnosis, this case did not show any characteristic findings of HCC on contrast-enhanced CT scan, but the T2-weighted image of Gd-EOB-MRI showed mildly high signal in the arterial phase, the hepatocellular phase showed a loose image, and diffusion-weighted imaging showed high signal, which led to suspicion of HCC. Therefore, the patient was suspected to have HCC. Moreover, the background of autoimmune hepatitis was a possible risk factor for HCC. Fine needle biopsy is one of diagnostic methods for histopathology, but there is a risk of peritoneal dissemination.20 Liver biopsy should be avoided as much as possible, and the authors decided on a partial resection of liver. Nowadays, laparoscopic surgery is performed as a safe and minimally invasive procedure.21,22
IHS was reported to present a variety of images in the literature. Varghese et al.23 reported that CT demonstrated ‘archiform’ enhancement pattern in the arterial phase and homogenous filling-in enhancement on portal venous and delayed phases.23 However, in another case, there was heterogeneous hyperenhancement in the arterial phase, isodensity in the portal venous phase, slight hypodensity in the delayed phase, and delayed enhancement of the capsule.24 CT imaging in each case is so varied that diagnosis can only be made with the use of other modalities. Gd-EOB-MRI is often used for differential diagnosis of hepatic malignancy; however, MRI could not rule out malignancy based on the findings of low signal in the hepatocellular phase and high signal in the diffusion-weighted image.25 There are some recent cases that were diagnosed with splenosis by MRI with other modalities that have been reported.26-28 Sonazoid contrast-enhanced ultrasonography has been reported to be useful in differentiating HCC. In the early arterial phase, the nodule was more enhanced compared with the surrounding liver parenchyma, and in the late vascular phase (Kupffer phase), the enhancement sustained compared with that of the surrounding area and did not show obvious defects as in HCC.29
Some cases have been reported where the use of Tc99m heat-denatured red blood cells scintigraphy allowed diagnosis confirmation, demonstrating phagocyte ability in the ectopic splenic tissue.30 Moreover, single-photon emission CT/CT after isotope administration increased specificity of planar imaging.31-35 However, Tc99m heat-denatured red blood cell scintigraphy has demonstrated superior sensitivity in the identification of residual or heterotopic splenic tissue, but preparation is complicated, and in vitro technique of isotope labelling is necessary. Kawada et al.36 reported a more convenient method using Tc99m sulfur colloid (conventional spleen scintigraphy), which is useful for differential diagnosis. Although this scintigraphy is easy and cost-effective, it is somehow difficult to distinguish between liver parenchyma and the ectopic splenic tissue. In this case, the patient did not have a history of splenectomy or abdominal trauma. IHS did not come to mind as a differential diagnosis, so splenic scintigraphy was not even considered.
In conclusion, IHS is a differential diagnosis that should be kept in mind when an atypically enhanced hepatic lesion is encountered in a patient with previous splenic trauma or splenectomy. It should be noted that IHS can occur even in very rare cases without splenectomy or abdominal trauma, as in the case presented here.







