Abstract
Acute fatty liver of pregnancy (AFLP) is an uncommon disorder affecting women in late pregnancy. It is increasingly recognised as an important cause of preventable maternal mortality across the world. The pathogenic mechanism of AFLP is now better understood; it appears that a compensated defective fatty acid oxidation becomes overt when metabolic stressors are superimposed on the increased energy demands of late pregnancy. The mother tends to rely more on fats as a source of energy in late pregnancy. This phenomenon may have an evolutionary basis and may explain why AFLP typically occurs in late pregnancy. The Swansea criteria have proven to be useful in early diagnosis of AFLP. Attempts to simplify these criteria further have proved helpful in early recognition of the disease. Although liver biopsy showing microvesicular steatosis of hepatocytes is the pathologic hallmark of AFLP, it is neither necessary nor safe in the antepartum setting. Current management strategies revolve around ensuring urgent delivery of the fetus and anticipating and managing complications of acute liver failure. While early recognition and multidisciplinary management have considerably improved maternal survival in AFLP, fetal outcomes remain poor. The authors postulate a therapeutic intervention to improve fetal outcomes in this disorder.
INTRODUCTION
Pregnancy-related disorders comprise several poorly recognised but important causes of liver dysfunction that complicate various stages of pregnancy. These include acute fatty liver of pregnancy (AFLP); haemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome; and pre-eclamptic liver dysfunction, which often results in poor maternal and fetal outcomes.1 With timely recognition, as well as early and aggressive management, the maternal mortality associated with these disorders can be mitigated. Recent advances have increased the current understanding of the pathogenesis of these disorders, which has translated into better overall management of these patients. This review focusses on the most under-recognised pregnancy-related liver disorder: AFLP.2 AFLP, first described in 1934, was initially termed (as per gross liver appearance on autopsy) acute yellow atrophy of the liver.3,4 AFLP is characterised by microvesicular fatty infiltration of the hepatocytes and presents in late pregnancy (late second or third trimester) with rapidly progressive illness.5
EPIDEMIOLOGY
AFLP is a rare disease, with a single large prospective population-based study (229 hospitals and 1,132,964 pregnancies in the UK) estimating an incidence of 5 cases per 100,000 pregnancies (3.8–6.5 per 100,000 pregnancies; confidence interval: 95%).6 The estimated incidence of AFLP was higher in hospital-based studies from the UK7 (114 cases per 100,000 pregnancies) and India8 (30 cases per 100,000 pregnancies). Furthermore, a retrospective study from a single hospital in India found that of 285 maternal deaths (in a total of 113,755 pregnancies from 1999–2011), 23 (8%) were secondary to pregnancy-related liver disorders and 17 (6%) had AFLP, histologically proven in 7 patients.9
PATHOGENESIS
The Timing of Acute Fatty Liver of Pregnancy: Clues from an Evolutionary Viewpoint
Why does this illness occur in an otherwise healthy woman in late pregnancy? The timing of this disease in late pregnancy may have an evolutionary basis. During periods of starvation, the human body switches from carbohydrate to fat stores as an energy source.10 Fat stores are the main and preferred energy source during prolonged starvation in hibernating animals (≤100 days hibernation duration) and in migratory birds during long distance, intercontinental, nonstop flights, which can cover 3,000–4,000 km.11,12 While the specific mechanisms regulating the transition to fat metabolism in migrating birds are unknown, recent studies have focussed on the role of peroxisome proliferator-activated receptors in regulating migratory adiposity.13 If these animals or birds experience a hindrance or blockage in utilising these fat stores, they could be expected to become energy deficient and fall sick during these annual periods of starvation.
In humans, the pregnant mother relies on fat stores as the main source of energy in late pregnancy.14 Why would the pregnant mother rely on fats as the primary energy source as the pregnancy advances? It is possible that fats are the preferred energy source for the mother to tide over the reduced dietary intake, as well as the markedly increased energy expenditure during labour (a situation similar to the migratory bird on a long distance flight that does not eat and experiences a markedly increased energy expenditure). It is also possible that the mother redirects dietary carbohydrates to enhance fetal nutrition. Thus, if the pregnant mother has some defect in metabolising or utilising her fat stores, she can be expected to become energy deficient in late pregnancy.15
AFLP is characterised by mitochondrial dysfunction causing liver failure, known as mitochondrial hepatopathy.5 Dysfunction of mitochondria, the power generators of the cell, leads to energy deficiency. In the cell, mitochondria are the sites where fatty acid oxidation takes place to produce ATP. They are also the site where fatty acid oxidation and glucose metabolism merge. A rat model of hepatic microvesicular steatosis (induced by sodium valproate) led to mitochondrial structural changes and oxidant stress in mitochondria and lysosomes of the liver.16
Fetal Fatty Acid Oxidation Disorders are Associated with Acute Fatty Liver of Pregnancy in the Mother
In some AFLP patients, fatty acid oxidation disorders (commonly defects of long chain 3-hydroxy acyl coenzyme A dehydrogenase [LCHAD]) in the fetus are noted. The enzymes involved in fatty acid oxidation are located on the inner mitochondrial membrane. Fetal fatty acid oxidation disorders are inherited in an autosomal recessive way with both parents being simple heterozygotes.
In a study comparing 50 infants with fatty acid oxidation disorders to 1,250 healthy control infants, maternal liver diseases (AFLP, HELLP syndrome, or pre-eclamptic liver dysfunction) were noted in 16.0% of fatty acid oxidation defect pregnancies compared with 0.9% observed in the control group (odds ratio: 20.4; 95% confidence interval: 7.8–53.2). Different types of fetal fatty acid oxidation defects were associated with maternal liver disease. While infants with LCHAD defects were 50-times more likely to be associated with maternal liver disease than controls, infants with short and medium chain fatty acid oxidation defects were 12-times more likely to be associated with maternal liver disease.17 Additionally, an analysis of 63 pregnancies in 18 mothers with a total of 28 LCHAD-deficient children noted AFLP, HELLP syndrome, and pre-eclampsia in 31%, and intrahepatic cholestasis in 10% of pregnancies, but in none of the pregnancies with a healthy fetus.18 Accumulation of acyl carnitine may be involved in pathogenesis of AFLP in these situations.19 However, other reports have shown a lack of association between fetal fatty acid oxidation disorders and maternal liver diseases.20,21
The Placenta as a Driver of Pathogenesis of Acute Fatty Liver of Pregnancy
The placenta shares a similar genetic profile to the fetus. Mitochondrial enzymes are expressed in the placenta. A dramatic improvement in the health of the mother with AFLP often occurs after delivery of the baby and the placenta. This observation led to studies on the placenta in AFLP patients.22 Raised levels of free fatty acids were seen in the placenta and serum of AFLP patients compared to controls; arachidonic acid and palmitic acid levels were higher in the placenta and serum of AFLP patients while oleic acid and myristic acid levels were higher in the placenta of AFLP patients. Serum arachidonic acid levels were 4-fold higher in AFLP patients (80 μm/mL) compared to healthy pregnant controls (20 μm/mL). Mitochondrial dysfunction and increased oxidative and nitrosative stress were also noted in the placenta and serum of AFLP mothers compared to controls.23
Arachidonic acid, at the concentration seen in serum of AFLP patients (80 μm/mL), induced mitochondrial dysfunction, oxidative stress in mitochondria, apoptosis (without necrosis), and fat deposition, suggestive of microvesicular steatosis, in Chang liver cell lines.23 Figure 1 summarises the authors’ current understanding of pathogenesis of AFLP.
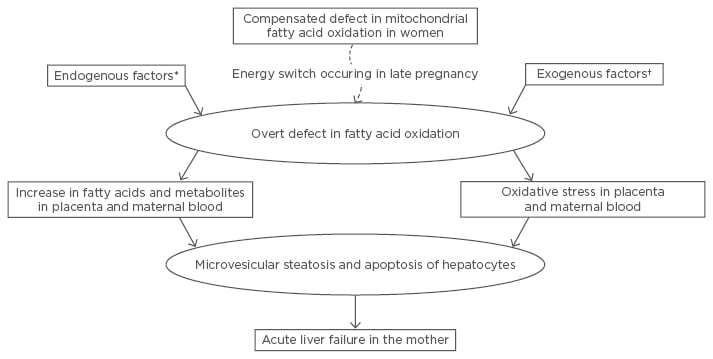
Figure 1: The authors’ current understanding of the pathogenesis of acute fatty liver of pregnancy.
There is an unmasking of a hitherto compensated defective fatty oxidation in women as a result of increased reliance on fats as an energy source during late pregnancy (energy switch). This is also precipitated by endogenous factors and/or exogenous factors.
*Fetus being homozygously defective in fatty acid oxidation or coexisting HELLP syndrome or pre-eclampsia; †For example, drug and intercurrent infection.
HELLP: haemolysis, elevated liver enzymes, and low platelets.
CLINICAL FEATURES AND INVESTIGATIONS
AFLP is typically limited to late pregnancy and the patient usually presents with vomiting, abdominal pain, and mild jaundice;24 symptoms of polydipsia and polyuria can be present but are rarely seen.
Though it is more common in primigravida, AFLP can occur in multiparous females with a history of prior uneventful pregnancies.2 The clinical course often progresses rapidly downhill, with eventual occurrence of encephalopathy, hypoglycaemia, and/or ascites. Rare complications include liver rupture and haemoperitoneum.2
At presentation, patients may or may not be clinically jaundiced with a mild-to-moderate increase in aminotransferases. Coagulopathy (prolongation of prothrombin time and/or hypofibrinogenaemia), renal failure, hyperuricaemia, hypoglycaemia, and leukocytosis are commonly observed at presentation or over the next few days. Ultrasound scan showing fatty acid infiltration of the liver is neither sensitive nor specific for the diagnosis of AFLP.24
The most specific, gold standard investigation to reach the diagnosis is liver biopsy demonstrating diffuse or perivenular microvesicular steatosis.25,26 Presence of coagulopathy and difficulties in performing liver biopsy in the antenatal mother are challenges that impact obtaining histological confirmation of AFLP in a suspected patient. Thus, liver biopsy is not advisable before swiftly embarking on management.27 Post-partum liver biopsy confirmation of AFLP may help in patient counselling (regarding further pregnancies) and individualising management of the child. The transjugular route provides a safe access point for liver biopsy in patients with coagulopathy.28 The microvesicular fatty infiltration of the hepatocytes is noted to rapidly resolve after delivery and thus liver biopsy, if undertaken, is preferably done within 4 days after delivery.27 The presentation of symptoms and signs and basic lab investigations form the basis of the Swansea diagnostic criteria for AFLP.
DIAGNOSIS
Realising the lack of diagnostic criteria for AFLP, Ch’ng et al.,7 based on retrospective analysis of multiple case series, proposed the Swansea criteria for AFLP diagnosis. These criteria are based on typical presentation, absence of an alternate explanation, and laboratory parameters noted in patients with AFLP.7 These criteria were later prospectively validated against a clinical diagnosis by Knight et al.6 In a retrospective study of 24 patients with suspected pregnancy-related liver disease, the Swansea criteria were validated against the gold standard for diagnosis, i.e., diffuse or perivenular microvesicular hepatic steatosis on liver biopsy, obtained in either the immediate postnatal (n=19) or post-mortem (n=5) period.27 Thus, antenatal application of the Swansea criteria (even without liver biopsy; i.e., presence of 6 of the 13 remaining criteria) is useful in diagnosing clinical AFLP and also predicts presence of microvesicular steatosis on liver biopsy.29 These diagnostic criteria have increased the ability to suspect AFLP (as they have very high negative predictive value) and to institute early management of these patients.30-32
The simplified criteria to diagnose AFLP states that the disease should be suspected in all women presenting in the late second or third trimester of pregnancy with unexplained acute liver failure (i.e., jaundice with coagulopathy often accompanied by encephalopathy and/or hypoglycaemia).33 The time required to evaluate other causes of liver failure has to be balanced against the urgency of delivery.
The cause(s) that needs to be evaluated depend on the aetiology of acute liver failure within that geographic locality and needs to be individualised for each patient (e.g., taking into account hepatitis A and E virus infection in endemic areas).34,35 Clinicians evaluate for drug-induced liver injury (by obtaining history of ingestion of potentially hepatotoxic drugs), malaria (peripheral smear examination), and acute viral hepatitis B before a diagnosis of AFLP is entertained.3,36
Overlap of Diagnosis with Other Pregnancy-Related Liver Disorders
In most patients, the diagnostic criteria for AFLP and HELLP syndrome show a considerable overlap.27,37 Typical changes on liver biopsy may help in differentiating these conditions. Whether this overlap is due to the non-specific nature of the diagnostic criteria or are secondary to overlap in pathogenesis is unclear and needs further clarification. Xiao et al.38 suggested that HELLP syndrome is caused by endothelial dysfunction leading to secondary thrombotic microangiopathy. It is likely that the initial event (either AFLP or HELLP) contributes additional stress that stimulates the other in a genetically predisposed individual (Figure 1). The management of disease likely remains unaltered, and so it may not be essential to make such a differentiation at present.
MANAGEMENT
Prompt suspicion, early recognition, and emergent careful delivery of the baby remain the cornerstones of management of patients with AFLP.4,39,40 Maternal survival is to be prioritised and any delay either in recognition of AFLP or in instituting delivery of the baby can adversely affect maternal outcome. It is important to recognise and categorise these patients as seriously sick and consider the intensive care or high dependency unit for disease management. The general principles guiding management in patients with acute liver failure (e.g., intravenous mannitol infusions for cerebral oedema) are also applicable for patients with AFLP, but these are beyond the scope of this article.
Constant involvement of various specialities, such as obstetrics, hepatology, intensive care, anaesthesia, haematology, and neonatology, is a must for optimal care of an AFLP patient. The mode of delivery is to be decided on a case by case basis by an obstetrician. Vaginal delivery possibly entails a longer delivery duration, as well as possible worsening of liver and/or multi-organ dysfunction, because added energy is needed for vaginal delivery in an AFLP patient who is already in a state of systemic energy deficit consequent to mitochondrial hepatopathy; in comparison, caesarean section poses an increased risk of bleeding and anaesthesia-related complications. Most centres prefer delivering the baby by caesarean section with careful anaesthetic management.41,42 Adequate blood product replacement to address coagulopathy is a prerequisite for both modes of delivery. Hypoglycaemia and postpartum haemorrhage are common complications that need to be anticipated;43,44 additionally, the use of broad-spectrum antibiotics as a prophylaxis should also be considered.
Intensive monitoring and continued management in the complication of acute liver failure is often required in the post-partum period. Occasionally, patients may require prolonged supportive management, including plasma exchange, and only rarely is liver transplant warranted.45-47 Managing AFLP patients demands an intensive multidisciplinary team approach, and thus early recognition and referral to an experienced and equipped centre is often required. Instituting early and aggressive management protocols (Figure 2) has reduced maternal deaths at most institutions across the world.2
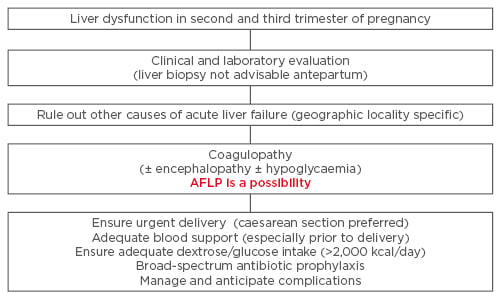
Figure 2: Management protocol in patients suspected to have acute fatty liver of pregnancy.
AFLP: acute fatty liver of pregnancy.
PROPOSED THERAPEUTIC INTERVENTION TO IMPROVE FETAL SURVIVAL IN ACUTE FAtty LIVER OF PREGNANCY
In AFLP, the postulated underlying defect is an inability to meet energy requirements in the face of a) the extremely high calorie requirements of late pregnancy (additional 500 kcals per day is typically required during the third trimester)48 and b) genetic defects restricting the rate at which fatty acid oxidation can proceed. The baby’s energy requirements cannot be met by beta oxidation of fatty acids, and therefore dextrose or glucose supplementations are necessary to counter the deficiency in substrate metabolism and energy release. We propose that upon diagnosis of AFLP, the mother should be fed with dextrose in amounts that provide the required 2,000 calories per day or more.48 This proposed intervention, aimed at improving fetal survival in AFLP, needs to be tested in clinical studies (Box 1).

Box 1: Basis for the proposed management strategy to reduce perinatal mortality in acute fatty liver of pregnancy.
Care of the Baby
The management of babies born to mothers who have AFLP needs specialised inputs from a neonatologist and clinical geneticist. The baby must be assessed for fatty acid oxidation defects.25,49 Specific fatty acid oxidation defects (e.g., E474Q mutation in LCHAD component of mitochondrial trifunctional protein) are noted in some populations.49,50 Some authors suggest universal screening for LCHAD deficiency (by acyl-carnitine assay) in all children born to mothers with AFLP,51 as LCHAD defects are the most common identified defects.52,53
The presentation of children with fatty acid oxidation defects can be extremely variable, from being asymptomatic to severely ill with encephalopathy, cardiomyopathy, or even sudden infant death. The symptoms tend to be precipitated by catabolic stress and all efforts are required to mitigate this complication at the earliest signs.54
Outcome
Most AFLP mothers show rapid improvement within a few days after delivery and on follow-up liver functions revert to normal.55 The prognosis in these patients is determined by the severity of liver dysfunction (serum bilirubin and prothrombin time), serum creatinine, and delay in delivery.56-58
Consequent to protocol-based urgent delivery (simplified criteria to diagnose AFLP), a steady decline in the contribution of AFLP and other pregnancy-related liver disorders to maternal mortality has been reported in one centre (maternal mortality due to pregnancy-related liver disorders decreased from 13% between 1999 and 2003 to 5% between 2004 and 2011).9 Similar results have been noted across various studies, with the maternal mortality due to pregnancy related liver disorders now expected to be <10%.2,4,5,59 The fetal outcome remains a challenge and improvement in management strategies may be required to address this important issue.
Future Pregnancies
There is a small but definite risk of recurrence during subsequent pregnancies, especially if there is a demonstrable defect in fatty acid oxidation.60,61 This should be discussed in detail with the patient.
CONCLUSION
Improved understanding of the pathogenesis of AFLP, early recognition using clinical diagnostic criteria, and aggressive management has resulted in improvement in maternal mortality in many centres across the world. Continued efforts are required to increase awareness regarding this preventable cause of maternal death. Further focus should be directed towards the improvement of fetal survival in mothers with AFLP.








