BACKGROUND
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the main cause of liver disease, and its incidence is rising due to the outbreak of metabolic syndrome and obesity. The spectrum ranges from simple steatosis to steatohepatitis, cirrhosis, and hepatocellular carcinoma, having become a common reason for liver transplantation.1,2
It is essential to stratify patients with MASLD according to the degree of liver fibrosis since its presence and progression determines the prognosis of the disease.2 Liver biopsy has traditionally been considered the gold standard for the diagnosis of cirrhosis and staging of fibrosis.1-3 However, it is an invasive procedure, carrying a risk of rare but potentially life-threatening complications.2,3 Therefore, a number of non-invasive markers have been developed over the past decade for the evaluation of liver fibrosis.1-3
AIM
This study aimed to analyse the usefulness of FibroScan, non-alcoholic fatty liver disease fibrosis score (NFS), aspartate aminotransferase to platelet ratio index (APRI), and fibrosis-4 index (FIB-4) indexes in evaluating the presence and severity of liver fibrosis in MASLD, using liver biopsy as the reference standard.
METHOD
The case records of 210 patients who underwent liver biopsy from March 2019–January 2023 were retrospectively reviewed, and for this investigation, those who had undergone FibroScan measurements and blood samples simultaneously or with a maximum difference of 3 months from liver biopsy were selected. Patients without histological confirmation of hepatic steatosis (defined as a liver fat content ≥5% on biopsy) were later excluded. NFS, FIB-4, and APRI were calculated. In all markers, indeterminate results were discarded. Liver fibrosis stage was determined using the meta-analysis of Histological Data in Viral Hepatitis (METAVIR) system.
RESULTS
A total of 194 patients were initially examined, and the presence of hepatic steatosis on biopsy was detected in 44.3% of those selected for the study. These patients had a mean age of 54.5±10.6 years, with 76.7% being females. FibroScan liver stiffness results ranged from 2.6–45.0 kPa (mean: 8.6 kPa). Serological marker values ranged from -5.2–1.3 kPa (mean: -2.1 kPa) for NFS, from 0.2–9.3 kPa (mean: 1.8 kPa) for FIB-4, and from 0.1–6.1 kPa (mean: 0.9 kPa) for APRI. In liver biopsy, 73.3% of the patients presented F0-1, while mild fibrosis (F≥2) was found in the remaining 26.8%. For advanced fibrosis (F3–F4), FibroScan showed a sensitivity of 64.3% and a specificity of 87.3%, with a positive predictive value of 50% and a negative predictive value of 92.5%. Its degree of concordance with the biopsy was moderate (kappa index [κ]: 0.46). NFS showed low sensitivity (25%) and high specificity (98.2%), having a weak degree of concordance with the biopsy (κ: 0.3). Regarding FIB-4 and APRI, both showed high sensitivity (85.7% for FIB-4 and 75% for APRI) and specificity (86.1% for FIB-4 and 81.2% for APRI), with a κ of 0.55 and 0.36, respectively.
CONCLUSION
The authors concluded that FibroScan and FIB-4 are comparable to liver biopsy with a moderate degree of agreement, being useful tools for the non-invasive detection of advanced fibrosis among patients with MASLD.
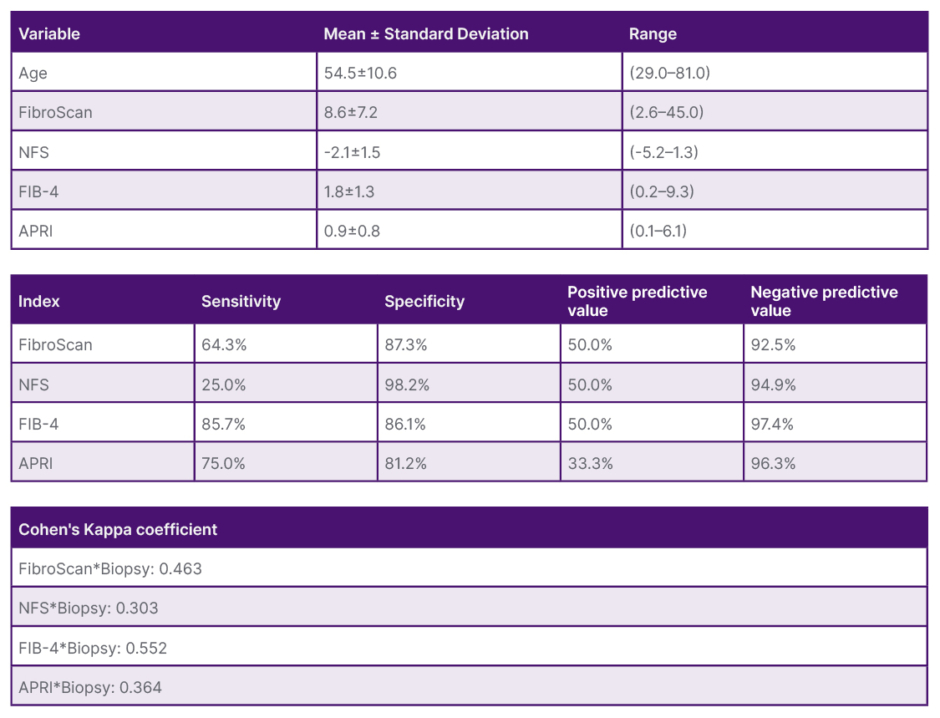
Figure 1: Suitability of non-invasive tests in the evaluation of liver fibrosis in patients with metabolic dysfunction-associated steatotic liver disease.
APRI: aspartate aminotransferase to platelet ratio index; FIB-4: fibrosis-4 index; NFS: non-alcoholic fatty liver disease fibrosis score.







