INTRODUCTION
Worldwide, 185 million people are infected with the hepatitis C virus (HCV);1 approximately 12% of these individuals have manifestations of decompensated cirrhosis.2 No studies have examined sustainable reductions in specific forms of decompensation in patients with HCV and decompensated cirrhosis after treatment. Thus, the primary aim of this study was to examine changes in the proportion of patients with HCV and decompensated cirrhosis with ascites, hepatic encephalopathy, and variceal bleeding at pretreatment compared to 3 and 12 months post sustained virological response (SVR). Secondary aims were to compare pretreatment and post-SVR Model for End-Stage Liver Disease (MELD) scores, Child–Pugh (CP) scores, and α-fetoprotein (AFP) levels.
METHODS
A retrospective chart review was performed on all patients with HCV and decompensated cirrhosis who began direct-acting antiviral (DAA) treatment between November 2014 and January 2016 at a tertiary medical centre. Measures of central tendency and frequency distributions were used for univariate analysis. Pretreatment proportions of patients with ascites, hepatic encephalopathy, and variceal bleeding were compared to 3 and 12 months post-SVR proportions using the McNemar–Bowker test. Changes in median MELD score, CP score, and AFP levels were compared using the Wilcoxon signed rank test.
RESULTS
Thirty-seven patients met the inclusion criteria. Most patients were male (57%), Caucasian (84%), treatment-naïve (60%), and genotype 1 (78%). The median patient age was 60 years. Ascites was resolved in 29% of patients at 3 months (65% versus 36%; p<0.01) and in 35% at 12 months (65% versus 30%; p=0.07). Hepatic encephalopathy was resolved in 54% at 3 months (70% versus 16%; p<0.01) and in 48% at 12 months (70% versus 22%; p=0.03). Variceal bleeding was resolved in 32% at 3 months (35% versus 3%; p<0.01) and in 27% at 12 months (35% versus 8%; p<0.01). Median AFP levels were significantly reduced (6.45 [range: 0.81–39.70] versus 4.24 [range: 0.60–15.70], respectively; p<0.01), with a median decrease of 2.21. There were no differences in statistical significance between pretreatment and post-SVR median (interquartile range) MELD scores (15 [8–28] versus 15 [6–36]; p=0.20) and median CP scores (7 [5–11] versus 7 [5–15]; p=0.60).
DISCUSSION
We demonstrated that patients who achieve SVR with DAA can also achieve reductions in manifestations of hepatic decompensation. These findings were clinically significant. Ascites and hepatic encephalopathy are the most common reasons for hospital admission among patients with advanced liver disease. Although there were no improvements in MELD and CP scores in this study, MELD and CP scores did not worsen with DAA treatment. This will allow clinicians to pursue DAA treatment in patients currently on liver transplantation waitlists with the potential benefit of improving morbidity and mortality without affecting their ability to obtain a transplant. Finally, the significant reductions in AFP scores after treatment can perhaps improve the clinical utility of AFP as a screening modality for hepatocellular carcinoma in patients with advanced liver disease.
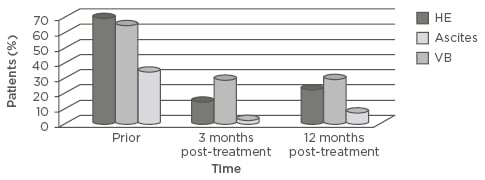
Figure 1: Manifestations of decompensated cirrhosis prior to treatment and 3 and 12 months post-sustained virological response.
HE: hepatic encephalopathy; VB: variceal bleeding.








