BACKGROUND AND AIMS
The definition and severity of checkpoint inhibitor-induced liver injury (ChILI) have been reported using the Common Terminology Criteria for Adverse Events (CTCAE) used in cancer therapy.1 Among patients with melanoma receiving checkpoint inhibitors (CPI), alanine aminotransferase elevation >5-fold (Grade 3) occurs in 1–16%.2 However, the incidence rate of ChILI by treatment duration (time at risk) per type of cancer and CPI regime has not been accurately assessed. The authors estimated the risk and incident rate of ChILI in patients with melanoma or renal cell carcinoma who received CPI at Nottingham University Hospitals, UK, from 2011 to 2021.
MATERIALS AND METHODS
Using a prospective oncology prescribing database, the authors identified all adult patients who received CPI for adjuvant or metastatic melanoma or metastatic renal cell carcinoma over 10 years (2011–2021). The authors performed prescription event monitoring using patient-related records and digital records to identify ChILI, other immune-related adverse events, and response to rechallenge. International Expert Working Group (EWG) for drug-induced liver injury case definitions were used to define ChILI cases and grade severity.3 Causality was assessed using Roussel Uclaf Causality Assessment Method (RUCAM).4 Duration at risk for each patient was calculated, and the risk and incidence rate of ChILI were measured.
RESULTS
Out of 432 patients, 47 patients met the criteria and were identified as possible ChILI. After causality assessment, 9/47 (19.1%) were excluded due to other aetiologies. Overall, ChILI occurred in 38 patients (8.8%) and the highest risk of ChILI was noted in patients with melanoma who received combination therapy (28.4%). RUCAM was possible in nine, probable in 22, and highly probable in seven cases. The overall incidence rate of ChILI was 11.5 (95% confidence interval: 8.2–15.8) per 1,000 patient-months, with most cases occurring in patients with melanoma treated with dual checkpoint inhibition (38.1; 95% confidence interval: 25.5–54.7), as shown in Table 1. The pattern of liver injury was hepatocellular in 22 cases (57.9%), mixed in 11 (28.9%), and cholestatic in five (13.2%). Although nine patients (23.7%) were classified with Grade 4 hepatitis according to CTCAE (life-threatening), ChILI severity was mild in 11 (29%) and moderate in 27 (71%) based on EWG grading. Only two patients developed jaundice, and none progressed to acute liver failure. Thirty-six of 38 patients (94.7%) received corticosteroids, with two requiring second-line therapy with mycophenolate mofetil. Mean time to resolution of ChILI was 82±65 days following steroid therapy compared with 49±21 days in those untreated. Of 38 ChILI cases, 15 (39.5%) were retreated with a single CPI, and only one patient developed recurrent ChILI (6.7%). However, three developed colitis (20%), and one each developed hypophysitis and severe neuropathy (6.7%).
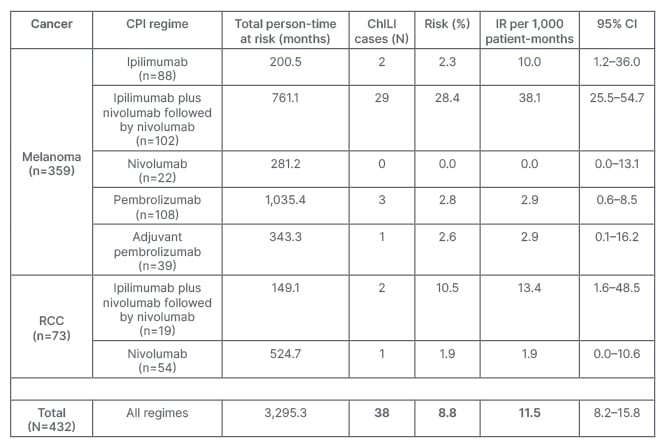
Table 1: Risk and incidence rate of checkpoint inhibitor-induced liver injury.
ChILI: checkpoint inhibitor-induced lung injury; CI: confidence interval; CPI: checkpoint inhibitor; IR: incidence rate; RCC: renal cell carcinoma.
CONCLUSION
Incidence rate of ChILI was 11.5 per 1,000 patient-months, with the highest incidence in patients receiving combination therapy for melanoma (38.1). Most cases were hepatocellular in pattern. Jaundice was rare, with no severe cases developing acute liver failure or requiring liver transplantation. Rechallenge with single CPI was feasible and most cases didn’t experience recurrence of ChILI; however, they were at risk of other immune-related adverse events, especially colitis. The risks and benefits of corticosteroid therapy need to be evaluated in a larger cohort of patients with ChILI.








