BACKGROUND AND AIMS
Approximately 3 million people have chronic hepatitis C virus (HCV) infection in the USA.1 However, there are limited data with regard to the effect of direct-acting antivirals (DAA), a treatment for HCV, on renal function. The purpose of this study was to examine changes in renal function after HCV treatment with DAA.
METHODS
This study was a single centre, retrospective analysis of patients with chronic HCV infection who were treated with DAA. Patients were included if they were seen in the clinic between December 2014 and December 2015, had confirmed diagnoses of chronic HCV infection by RNA polymerase chain reaction test, and achieved sustained viral responses after treatment. Post liver transplantation patients were excluded, as were patients with incomplete data. Data were collected on demographics, presence of cirrhosis, presence of hepatic decompensation, presence of hepatocellular carcinoma, genotype, treatment experience, treatment regimen and duration, and pre and post-treatment glomerular filtration rate (GFR) and serum creatinine. Measures of central tendency and frequency distributions were used for univariate analysis. The paired sample t-test was used to compare serum creatinine levels before and after treatment.
RESULTS
A total of 306 patients were included in the study. Patient characteristics included a mean age of 57 years, 53% male, 70% non-Hispanic white, 84% HCV genotype 1, 70% treatment-naïve, 49% with cirrhosis, 14% with hepatic decompensation, and 5% with hepatocellular carcinoma. Eighty-three percent of patients received ledipasvir or sofosbuvir. The mean baseline serum creatinine level was 0.96, and 90% of patients had normal baseline renal function. Of those with normal baseline renal function, GFR stayed the same in 97% of patients and worsened in 3%. Of those with baseline renal impairment, 23% of patients had improvement in GFR, 68% had stable GFR, and 10% hadworsened GFR. Altogether, GFR improved or stayed the same in 96% of patients (Table 1). There were no statistically significant differences between serum creatinine levels before and after treatment (0.96 versus 1.00; p=0.15).
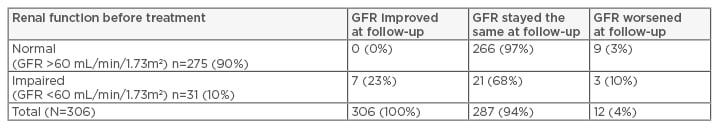
Table 1: Proportion of hepatitis C patients treated with direct-acting antivirals with renal improvement, no change, or worsening at 3–6 months post-sustained virological response (N=306).
GFR: glomerular filtration rate.
CONCLUSION
In this study, DAA have been shown to be safe from a renal function standpoint. Furthermore, nearly one-quarter of patients in this study with baseline renal impairment had improvements in renal function after DAA treatment. Early DAA treatment may not only prevent chronic liver disease but also prevent and/or treat chronic renal insufficiency. Larger prospective studies are needed to further investigate these findings.








