Presenter: Courtney DiNardo
University of Texas MD Anderson Cancer Center, Houston, Texas, USA
Disclosure: Dr DiNardo has received research support (to institution) from AbbVie, Agios Pharmaceuticals, Calithera Biosciences, Celgene, and Daiichi Sankyo; and consultation and participation in advisory boards for AbbVie, Agios Pharmaceuticals, Celgene, Daiichi Sankyo, Jazz Pharmaceuticals, ImmuneOnc Therapeutics Inc., Novartis, and Notable Labs Inc.
Acknowledgements: Medical writing assistance was provided by Dr George Xinarianos, OncoMed Communications & Consultancy Ltd, London, UK.
Support: The publication of this article was funded by Agios Pharmaceuticals.
Citation: EMJ Rheumatol. 2020;8[1]:50-53.
Summary:
Somatic mutations in the IDH1 gene are relatively common, occurring in 6–14% of patients with acute myeloid leukaemia (AML), and are especially enhanced in populations such as the elderly.1-3 Ivosidenib is a first-in-class, oral, targeted inhibitor of the mutant isocitrate dehydrogenase 1 (mIDH1) enzyme. It is approved in the USA for the treatment of AML with a susceptible IDH1 mutation, as detected by a U.S. Food and Drug Administration (FDA)-approved test in adults with newly diagnosed (ND) AML who are ≥75 years of age or who have comorbidities that preclude the use of intensive induction chemotherapy (IC), and in adults with relapsed or refractory (R/R) AML.4-6 A recent report from an ongoing Phase Ib study of the ivosidenib plus azacitidine combination, for the treatment of patients with mIDH1 ND AML ineligible for IC, showed a complete remission (CR) plus CR with partial haematologic recovery (CRh) rate of 70% and demonstrated a favourable safety profile in these difficult-to-treat IC-ineligible patients.7 AGILE is an ongoing Phase III study of the ivosidenib plus azacitidine combination regimen in adults with mIDH1 ND AML.8
Venetoclax is a potent, selective, oral inhibitor of B-cell lymphoma-2. It is approved in the USA in combination with azacitidine, decitabine, or low-dose cytarabine for the treatment of ND AML in adults who are ≥75 years of age or who are IC-ineligible.9 Azacitidine reduces DNA methylation by inhibiting DNA methyltransferases, and as a monotherapy has shown promising clinical activity in older patients with ND AML.10 The combination of venetoclax plus azacitidine in treatment-naïve, elderly patients with mIDH1/2 AML showed a CR/CR with incomplete haematologic recovery (CRi) rate of 71%.9
In vitro studies of mIDH1R132H erythroleukaemia cell lines treated with ivosidenib and azacitidine have shown enhanced cell differentiation and potentiation of apoptosis compared to either agent alone.11
The authors report a primary analysis of ivosidenib (500 mg daily, Day 15-continuous) combined with venetoclax (Days 1–14 per 28-day cycle), with or without azacitidine (75 mg/m2, Days 1–7). The dual primary objectives of this Phase Ib/II, open-label, nonrandomised study12 were to assess the safety and efficacy of ivosidenib in combination with venetoclax, with or without the addition of azacitidine, for the treatment of mIDH1 myeloid malignancies. Eligible patients were enrolled into one of three successive cohorts (Cohort 1: ivosidenib plus venetoclax 400 mg; Cohort 2: ivosidenib plus venetoclax 800 mg; Cohort 3: ivosidenib plus venetoclax 400 mg plus azacitidine). Primary endpoints included safety and tolerability and overall response rate, measured by the International Working Group (IWG) response criteria.13
Twenty-one patients (median age: 67; 12 [57%] male) were enrolled. Seventeen patients had AML: nine with R/R AML, five had treatment-naïve AML, and three had hypomethylating agent-failure myelodysplastic syndrome, with progression to secondary AML. Four patients had high-risk myelodysplastic syndrome.
The most frequent (≥15%) Grade 1/2 adverse events (AE) were diarrhoea (75%), nausea (30%), vomiting (25%), and differentiation syndrome (15%). The most frequent (≥15%) Grade 3/4 AE included pneumonia (70%), febrile neutropenia (50%), and abdominal pain (15%). AE of special interest included differentiation syndrome (n=4; Grade >3 in 1) and tumour lysis syndrome (TLS) (n=2), including one Grade 3 TLS event in a NPM1+ co-mutated patient (successfully managed without haemodialysis). No patients died within 60 days of starting treatment, but one death was reported in the study because of febrile neutropenia in the setting of persistent disease. One patient, who had one kidney, had dose-limiting toxicity of TLS. Median cycle lengths for Cycle 1 were 27.0 days, 28.0 days, and 39.5 days for Cohort 1, Cohort 2, and Cohort 3, respectively; and for Cycle 2 were 28.0 days, 29.0 days, and 45.0 days for Cohort 1, Cohort 2, and Cohort 3, respectively.
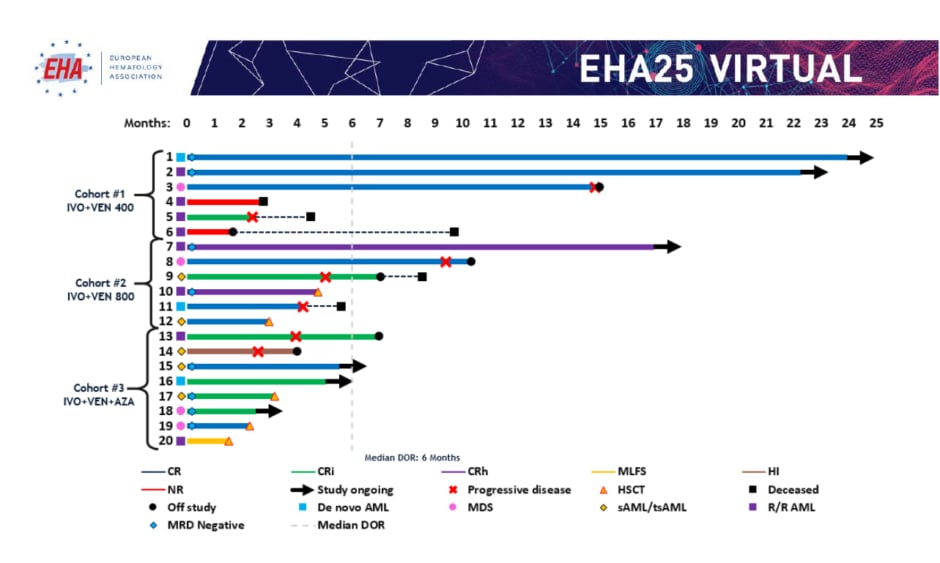
Response rates are summarised in Table 1. In evaluable patients (n=20), composite CR ([CRc]: CR+CRi+CRh) rates were 80% overall (de novo AML: 100%; secondary AML/treated-secondary AML: 80%; R/R: 63%), and were 67%, 100%, and 75% by cohort, respectively (median time to best response: 2 months). Fifty percent of patients who achieved CRc were also minimal residual disease (MRD) negative by flow cytometry. One patient experienced a morphological leukaemia-free state and one patient experienced haematological improvement without CR/CRi. Of the 20 evaluable patients, six (30%) remained on study, five (25%) proceeded to stem cell transplantation (four in CR and one in a morphological leukaemia-free state), two (10%) were nonresponders, and six (30%) experienced progressive disease after a median of 3 months in CRc (Figure 1).14 With a current median follow-up time of 7 months, the median event-free survival was 9.4 months for all patients. Molecular profiling showed a diverse molecular landscape across patients. Active signalling mutations (NRAS, KRAS, FLT3-ITD/TKD, PTPN11, and NF1) were detected in eight patients (40%). Sixty-six percent of patients without response or with relapse had active signalling mutations and were associated with treatment resistance. The median duration of response in patients with and without active signalling mutations was 1.6 months and 11.9 months, respectively (p=0.043). Median overall survival was 8.5 months in patients with active signalling mutations and was not reached in patients without active signalling mutations (p=0.02).
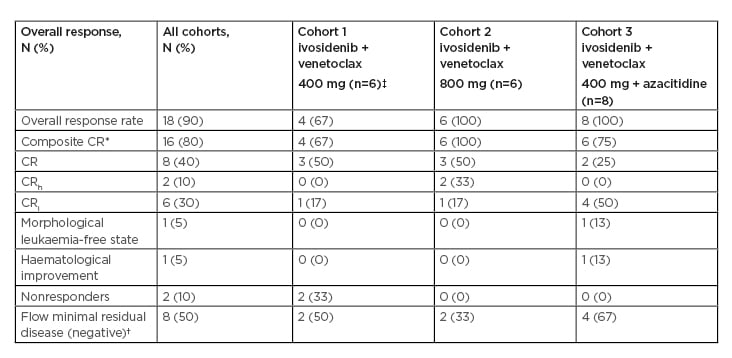
Table 1: Response rates.
*CRh and CRi represented as mutually exclusive.
†Among patients achieving a composite CR.
‡One patient in Cohort 1 was unevaluable and replaced.
CR: complete remission; CRh: complete remission with partial haematologic recovery; CRi: complete remission with incomplete haematologic recovery.
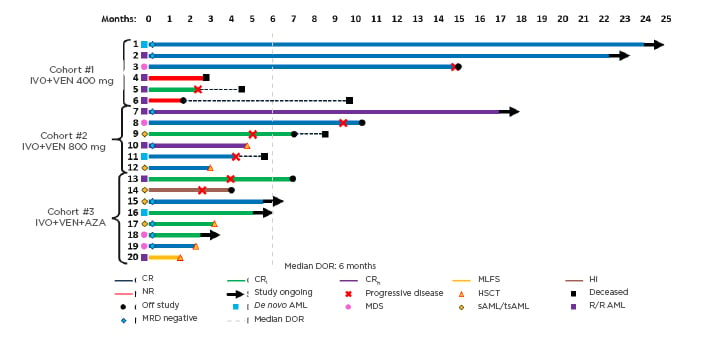
Figure 1: Treatment duration, best overall response, and minimal residual disease.
AML: acute myeloid leukaemia; AZA: azacitidine; CR: complete remission; CRh: complete remission with partial haematologic recovery; CRi: complete remission with incomplete haematologic recovery; DOR: duration of response; HI: haematological improvement; HSCT: haematopoietic stem cell transplantation; IVO: ivosidenib; MDS: myelodysplastic syndrome; MLFS: morphological leukaemia-free state; MRD: minimal residual disease; NR: nonresponders; sAML/tsAML: secondary acute myeloid leukaemia/treated-secondary acute myeloid leukaemia; R/R: relapsed or refractory; VEN: venetoclax.
Adapted from DiNardo CD et al.14
In patients with CR, with a current median follow-up of 2.5 months, duration of response was 3.0 months in MRD-positive patients and was not reached in MRD-negative patients. Median event-free survival was 5.0 months in MRD-positive patients and was not reached in MRD-negative patients; median overall survival was not reached in either group.
Overall, these preliminary data demonstrated that the combination of ivosidenib plus venetoclax with or without the addition of azacitidine was both well tolerated and effective. It was shown that these molecularly targeted combination regimens are effective for advanced mIDH1 myeloid malignancies, with a CRc in 80% of patients. Undetectable MRD by flow cytometry was found in 50% of patients with CR, with responses currently ongoing. In the triplet combination cohort, undetectable MRD by flow cytometry was found in 67% of patients with CR, with responses currently ongoing. This combination regimen was well tolerated, with a manageable toxicity profile. Treatment cycles longer than 28 days are required with the triplet combination for managing cytopenias. Additional follow-up and accrual in the Phase Ib part of the study is currently ongoing and thus, determination of the recommended Phase II dose and more mature efficacy, tolerability, and biomarker data are forthcoming. In the future Phase II part of the study, the authors aim to confirm efficacy in two cohorts (n=20 each) of treatment-naïve and R/R mIDH1 patients treated with ivosidenib plus venetoclax, with or without the addition of azacitidine.