Presenters: Pietro Quaglino,1 Martine Bagot2
1. Department of Medical Sciences, University of Turin, Italy
2. Department of Dermatology, Saint-Louis Hospital, Université de Paris, France
Disclosure: Quaglino is on the advisory boards of and has received speaker fees from 4SC, Celgene, Helsinn Recordati, Innate Pharma, Kyowa Kirin, Takeda, and Therakos. Bagot is on the advisory baords of and has received speaker fees from Galderma, Helsinn Recordati, Innate Pharma, Kyowa Kirin, and Takeda.
Acknowledgements: Eleanor Roberts, Beeline Science Communications Ltd., London, UK.
Disclaimer: The opinions expressed in this article belong solely to the named authors.
Support: The writing and editorial support was funded by Kyowa Kirin International.
Citation: EMJ Hematol. 2021;9[Suppl 7]:2-10.
Summary
Mycosis fungoides (MF) and Sézary syndrome (SS) are two types of cutaneous T cell lymphoma (CTCL) that primarily present in the skin. However, as there can be extracutaneous manifestations, the assessment of blood tumour burden, as well as of lymph node and visceral organ involvement, is crucial for accurate staging of these conditions. Such staging is used to best direct patient management that, in people with extracutaneous involvement, usually necessitates systemic therapy. These can be brentuximab vedotin and mogamulizumab, both where licensed, which have been shown in a number of clinical trials and real-world studies to be especially beneficial for those with blood involvement. Extracorporeal photopheresis (ECP) and alemtuzumab have also been shown to be useful, although studies are limited. At the European Organisation for the Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Group 2021 congress, Pietro Quaglino, Associate Professor of Dermatology, Department of Medical Sciences, University of Turin, Italy, and Martine Bagot, Head of the Department of Dermatology, Saint-Louis Hospital, Université de Paris, France, discussed blood involvement in CTCL pathogenesis, and the use of systemic therapy to treat MF and SS.
Introduction
CTCL is a rare type of non-Hodgkin lymphoma, primarily presenting in the skin but with extracutaneous involvement in some patients. Around 60% of cases of CTCL are classed as MF, with around 10% classed as SS.1 MF most often presents as polymorphic skin patches or plaques, which may evolve into tumours and erythroderma in around one-third of patients.2 SS presents with pruritic erythroderma, lymphadenopathy, and Sézary cells in the skin, blood, and lymph nodes, which are clonally related neoplastic T cells with cerebriform nuclei.1
At the EORTC Cutaneous Lymphoma Group 2021 congress, Quaglino discussed the role of blood involvement in the pathogenesis of CTCL, and Bago discussed the real-world experience using systemic therapy to treat blood involvement in CTCL.
Blood–Skin Dynamics in Mycosis Fungoides and Sézary Syndrome
While MF and SS may, according to Quaglino, be confined to the skin during the earlier disease stages, he stressed: “It is important to remember that this disease has the potential to spread outside of the skin.” Patches can develop into plaques, and later develop into tumours and erythroderma.1
MF was previously though to arise from a founding tissue-resident mature memory T cell. However, this does not account for why skin lesions arise multifocally, rather than at a single location, and some other features of MF that are incompatible with malignant transformation of skin-resident T cells.3
More recently, a study proposed a model of MF pathogenesis, wherein neoplastic T cell clones circulate in the peripheral blood from early in the disease and consecutively seed skin lesions, leading to their growth and evolution. Clones that have particularly high proliferative capacity in the skin may then re-enter the circulation, and seed other dermal areas.3,4 These clones in the blood could, suggested Quaglino, “become a target for treatment, as well as biologic prognostic indicators in this disease.”
This model could also explain why depletion of skin-resident T cells with total skin electron beam therapy or psoralen plus ultraviolet A therapy may provide a short-term response, but generally does not impact long-term remission.3
Mycosis Fungoides and Sézary Syndrome as Multicompartmental Conditions
As there may be extracutaneous involvement in MF and SS, they are considered multicompartmental conditions. As such, assessment of MF and SS should include not only the skin but also lymph nodes, viscera, and blood, even in early phases of the disease.5,6
Management of MF and SS is stage dependent, so accurate staging is essential.7 Compartmental involvement is the most important prognostic factor in MF and SS, and drives disease staging.1,8 As detailed in Table 1, ‘early stage’ comprises disease stages IA-IIA and is largely driven by the type and extent of skin involvement, though blood involvement and lymph node involvement are seen in around 21% and 16% of early-stage patients respectively. ‘Advanced stage’ describes disease Stages IIB-IVB and sees blood class drive disease stage in erythrodermic (T4) patients. Lymph node effacement and visceral involvement are reserved for the most advanced stages.7

Table 1: International Society for Cutaneous Lymphomas-European Organisation for Research and Treatment (2007) disease staging in mycosis fungoides and Sézary syndrome.
Adapted from Scarisbrick, 2018.7
B: peripheral blood; M: visceral organs/metastasis; N: lymph nodes; T: tumour/skin.
While high (B2) level blood involvement occurs in advanced stages,5 it is also of note that lower (B1) level involvement can occur in 15–26% of people in early-stage MF.9 This, discussed Quaglino, “means that one out of five patients in early-stage diseases could have initial, limited blood involvement.” Therefore, pointed out Quaglino, in the early stages, blood involvement still needs to be diagnosed, and followed up accordingly.
A large retrospective analysis of 1,502 patients with MF and SS previously found significant increased risk of disease progression and decreased median survival times (p<0.001) from around 18–25 years in those with no blood involvement, to around 3 years in those with any level (B1 and B2) of blood involvement.10 “What we don’t know clearly though,” said Quaglino, “is the prognostic relevance of blood involvement in early phases of the disease.” A molecular analysis study (n=258 early-stage patients) found that even low-level blood involvement (as indicated by T cell receptor gene arrangement and flow cytometry) was correlated with significantly worse overall survival, compared to no blood involvement (p=0.046). This may suggest blood involvement is in fact related to a particular clinical course.11 However, stressed Quaglino, the full impact of B1 blood involvement on early phases of the disease is yet to be fully elucidated.
Assessment of Blood Tumour Burden
According to EORTC-Cutaneous Lymphoma Task Force 2018 recommendations, blood tumour burden (blood involvement) is best assessed using flow cytometry to determine absolute counts of CD4+/CD7− or CD4+/CD26− cells.12 This, stated Quaglino, is a “homogenous, well-structured, reproducible way for determining blood involvement,” and can be used for diagnosis, to track disease progression, and to assess response to therapy. Using this system, B0 means there are <250 CD4+/CD7− or CD4+/CD26− cells/μL present, B1 means there are 250−999 cells/μL, and B2 means there are ≥1000 cells/μL.13
Quaglino reported that while his clinic carries out flow cytometry on all patients at first diagnosis,13 the presence of early stage B0/B1 blood involvement would not change treatment options at this time. In those who initially present with early-phase disease, he reported that they would repeat flow cytometry if there were clinical suggestions of disease progression. Having flow cytometry results at early stages, discussed Bagot, may be useful for potential future research to track blood involvement.
“The situation is completely different for erythrodermic patients,” Bagot highlighted, “where it is extremely important to do flow cytometry because […] if they have blood involvement, they need systemic and not only skin-directed therapy.”
Treatment Options for Patients with Extracutaneous Involvement
The most important driver for treatment choice, said Quaglino, is blood and skin involvement. The 2021 National Comprehensive Cancer Network (NCCN) guidelines state that patients with any level of blood involvement (B1−2), even where MF is otherwise considered early stage, should be considered for systemic treatment, as appropriate for the treatment of Stage III disease.14
In patients with erythrodermic, advanced stage, or treatment-refractory early-stage disease, systemic therapies may be used in an escalating fashion, according to need.15 These include ECP alone; ECP plus ultraviolet A and plus retinoids or interferon α; mogamulizumab; monochemotherapy (gemcitabine or pegylated doxorubicin, though not as first-line); brentuximab vedotin; alemtuzumab (not currently licensed for use in patients with MF and SS); and allogenic stem cell transplant (particularly in young patients).6,14,16
Extracorporeal photopheresis
ECP was one of the first systemic therapies used for patients with MF and SS, discussed Bagot. Here, blood is pumped outside the body in a loop and exposed to the photoactivated drug 8-methoxypsoralen.16 A review of trials and case series, which included patients from Stage IB to Stage IV, showed an overall response rate (ORR) of 31−86%, and a complete response (CR) of 0−33%.17 There is no current evidence to support its use in early-stage disease.17,18
Patients with erythrodermic Stage III-IVA1 MF and SS may benefit from regimens that combine ECP with systemic therapy, such as interferon α.19,20 “We know that ECP has a very strong safety profile,” relayed Bagot. Indeed, studies have shown that there are usually minimal adverse events with ECP, and no reports of World Health Organization (WHO) Grade III−IV side effects to date. A small number of patients have experienced hypotension during treatment only, and mild anaemia and/or thrombocytopaenia following therapy.21 “On the whole though,” reported Bagot, “it is a very efficient treatment, and very well tolerated.”
Brentuximab vedotin
The antibody-drug conjugate brentuximab vedotin, which combines an anti-CD30 monoclonal antibody and the microtubule agent monomethyl auristatin E, is licensed for use in adults with CD30+ CTCL who have had at least one prior systemic therapy. It can be used in CTCL patients for up to 16 cycles.22 A retrospective, multicentre, real-world analysis of 67 people with MF and SS showed an ORR of 67.2%. Compartmental response in blood was specifically investigated in just 10 patients (none with SS), where the response rate was reported as 40.0%.23,24
In this study, the most common adverse event was peripheral neuropathy (44%), which was reversible in the majority of patients (88%). There was a statistically significant association between peripheral neuropathy development and the number of brentuximab vedotin infusions (p=0.017).24
Alemtuzumab
The anti-CD52 monoclonal antibody alemtuzumab binds to CD19+ B lymphocytes and CD3+ T lymphocytes, and at lower levels on natural killer cells, monocytes, and maacrophages.23 It is currently licensed for use in people with multiple sclerosis23 but, discussed Bagot, though it is not currently licensed for MF and SS, it may exceptionally be available for such patients due to its ability to deplete circulating lymphocytes. A Phase II trial delivered this drug to 22 patients with advanced MF and SS and showed an ORR of 55%, with a median time to treatment failure of 12 months.25 Case series (Stages IIB−IV) of standard- or low-dose alemtuzumab have shown ORRs of 37–100%, and a CR of 21–47%.17
However, as a number of T and B lymphocytes are destroyed with this treatment, immunosuppression is common, with 50–71% of patients experiencing infectious complications.23,25 Up to 39% experienced autoimmunity23,26 and haematological toxicities in the Phase II trial, including anaemia (95% Grade 0−1 and 5% Grade 2−3); neutropenia (77% Grade 0−1, 5% Grade 2−3, and 18% Grade 4 after 8−12 weeks treatment); and thrombocytopaenia (82% Grade 0–1, 13% Grade 2−3, and Grade 4).25
Mogamulizumab
Mogamulizumab is a humanised IgG1 κ monoclonal antibody that is licensed for use within the European Union, USA, and Japan. The Phase III MAVORIC trial of mogamulizumab, the largest randomised study of systemic therapy in CTCL, compared it with the histone deacetylase inhibitor vorinostat in adults with Stage IB−IVB MF and SS, who had an Eastern Cooperative Oncology Group (ECOG) performance score ≤1, and had undergone at least one prior course of systemic treatment. While mogamulizumab targets C-C chemokine receptor type 4, which is overexpressed on malignant T cells in CTCL, its expression was not an inclusion criterion.27
In this trial, patients were randomised to either once weekly (for the first 28 days), then once every 2 weeks 1 mg/kg intravenous mogamulizumab (n=186), or 400 mg/day oral vorinostat (n=186) for 28-day cycles. Participants were stratified by CTCL subtype (MF or SS) and disease stage (IB−II versus III−IV). If any patients in the vorinostat group progressed, or there was intolerable toxicity after two 28-day cycles, they could crossover to the mogamulizumab group.27
Flow cytometric analysis of blood tumour burden was carried out after each treatment cycle27 as per EORTC recommendations,13 and post hoc analyses of the effects of mogamulizumab, according to baseline blood tumour burden, have been published.28
In terms of the reduction of blood tumour burden itself, results showed that in both those classed as B1 or B2, there was a rapid and prolonged decrease of malignant cell counts with mogamulizumab, which fell to be similar to those with B0 blood involvement after the first treatment cycle, and remained so for all subsequent cycles (11 cycles for those initially B1, 22 cycles for those initially B2).28
Further, skin response, measured as the change in modified severity-weighted assessment tool score, decreased across all B-classes after the first cycle, and were at least 50% lower than baseline by the twelfth cycle. Bagot discussed the correlation between baseline B-class and the response in the skin, with the percentage reduction from baseline being greatest in those classed as B2, and least throughout the study in the B0 group, though this had approached that of the B1 group by the end of the study.28
Health-related quality of life is an important factor to consider in patient care. One issue that may impact people with MF and SS is the occurrence of pruritis, which can increase with intensity as the disease progresses.29 The ItchyQoL scale is a pruritis-specific quality of life instrument,30 used in the MAVORIC trial to ascertain if baseline blood involvement impacted mogamulizumab effect on the health-related quality of life. Analysis found that from Cycle 1, there were improvements from baseline in all groups in the ItchyQoL functional limitation domain, including impacts on interactions with others, sleep, intimacy, and concentration. This was statistically significant at cycles 2, 6, 7, 9, 11, and 12 in mogamulizumab-treated patients with blood involvement.31
Bagot highlighted a number of other efficacy endpoints that were investigated in post hoc analyses of the MAVORIC data by patient blood tumour burden. Progression-free survival (PFS) overall was statistically significantly greater with mogamulizumab, compared to vorinostat in MAVORIC (Table 2). PFS, ORR, and time to next treatment were statistically significantly greater with mogamulizumab for the B2 population for all outcomes, and for PFS and time to next treatment for the B1 population (p<0.0001 in all cases). This highlights a trend to greater efficacy in patients with blood involvement treated with mogamulizumab (Table 2).28
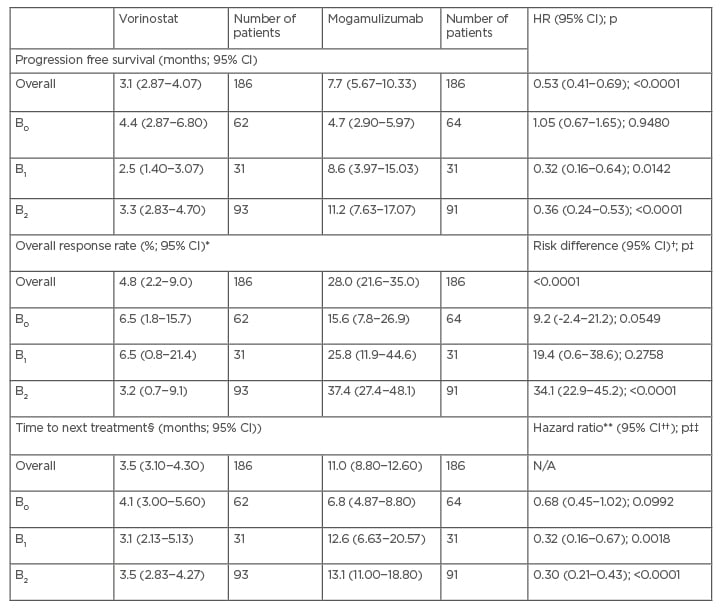
Table 2: Progression free survival, overall response rate, and time to next treatment by blood burden in post hoc analysis of the MAVORIC trial.
*95% CI for response rate is the exact 95% CI.
†Risk difference (i.e., attributable risk) is excess risk of a patient achieving an overall response with mogamulizumab versus vorinostat. The 95% CI for risk difference is the exact 95% unconditional CI for the risk difference (mogamulizumab−vorinostat).
‡P values were obtained from a Cochran–Mantel–Haenszel test adjusting for disease type, disease stage, and region.
§TTNT is defined as time from randomisation to date of first new systemic therapy. Mogamulizumab, used as the crossover drug, is regarded as systemic therapy. Patients who did not receive any subsequent therapy were censored at last survival follow-up.
**HR and 95% CIs were based on the Cox proportional hazards model with treatment, disease type, disease stage, and region as covariates.
††95% CIs were obtained from the SAS LIFETEST Procedure using log-log transformation.
‡‡P values (two-sided) were obtained from a stratified log-rank test with disease type, disease stage, and region as stratification factors.
Adapted from Cowan et al., 2021.28
CI: confidence interval; HR: hazard ratio.
Real-world studies of mogamulizumab
One retrospective observational study (OMEGA) of the efficacy and safety of mogamulizumab included 124 patients with MF or SS in France. These patients were predominantly Stage IVA1 (49.6%), with 21.8% having early-stage disease.32
Here, the best ORR (CR and partial response) was 59% in MF and SS combined, 47% in patients with MF, and 68% in patients with SS.32 Interestingly, these percentages were all higher than observed in the MAVORIC trial (28%, 21%, and 37%, respectively);27 however, of note, Bagot explained that due to differences in studies these are not directly comparable. While CR and partial response were more frequent in patients with SS (20% and 48%, respectively) compared to patients with MF (4% and 43%, respectively), stable disease was more common in those with MF compared to SS (43% and 27%, respectively).32
Assessment of blood response in the total population showed that this occurred in 55% of patients (with 68% being shown in MAVORIC),27 with seven of eight (88%) patients with B1-MF, and 31 of 54 (57%) patients with B2-SS achieving B0 as their best response. Figure 1 shows that when global response was assessed by blood involvement, there was a positive correlation with ORR, with ORR increasing as blood involvement increased.32

Figure 1: Global response in patients with mycosis fungoides and Sézary syndrome by blood involvement (n=111).32
B: blood; CR: complete response; ORR: overall response rate; PD: progressive disease; PR: partial response; SD: stable disease.
“It is interesting to see,” pointed out Bagot, “that the patients with B2 had a higher ORR than those with B0, and there is an interesting ORR in patients with B1.” Most of the latter, she continued, “were erythrodermic patients, so could be considered as pre-SS.”
Adverse events in the OMEGA study occurred in 54% of patients (168 individual events), none of which were severe, explained Bagot.32 These were predominantly skin and subcutaneous disorders, including mogamulizumab-associated rash (MAR) (27%); blood and lymphatic disorders (23%); and general disorders or anomalies at the administration site (18%). In total, seven patients died, five of disease progression and two of nontreatment-related sepsis.32
Mogamulizumab-associated rash
One of the most common adverse events to occur following mogamulizumab administration is MAR, observed in 24−68% of patients.27,33 Clinical presentation can be heterogenous, explained Bagot, and can include spongiotic/psoriasiform dermatitis, lichenoid/CD8+ interface dermatitis, and granulomatous dermatitis. There are four main clinical patterns of MAR: morbilliform eruption or erythroderma; MF-like papules and/or plaques; photoaccentuated dermatitis; and folliculotropic MF-like presentation, predominantly in the head and neck.34
MAR may be misinterpreted as disease progression; however, there are several characteristics that can help to distinguish the two following skin evaluation, skin biopsy, and blood analysis. These include an absence of T cell receptor clonality and CD4:CD8 ratio reduction or inversion, which are more strongly associated with MAR.33-35 To expand on this, Bagot discussed how “one good indicator is the clone. If the patient has progression that escapes mogamulizumab, the clone will progress in the skin and in the blood. Also in the blood,” she continued, “you may have an increase of the tumour population. In contrast, in MAR, the T cell clone disappears in the skin and in the blood.”
Of interest, higher blood tumour burden may make both clinical response to mogamulizumab and MAR more likely. A retrospective analysis (n=24) found that in those with MAR, the ORR was much higher at 88%, compared with 28% in those without MAR.33 Similarly, in a study of patients with relapsed or refractory MF (n=105) or SS (n=79), the patients with SS showed a response rate of 56% in 25 patients with MAR, and 30% in 54 patients without MAR, a significant difference (p=0.002). In the 19 patients with MF and MAR, there was a numerical, but not significant difference response rate, compared to those without MAR.36
Treatment for MAR, advised Bagot, should be directed by rash severity with treatment for Grades 1 and 2 MAR usually being a topical corticosteroid. If MAR is Grade 3, treatment with mogamulizumab should be interrupted, and the rash managed appropriately. In those patients in whom the rash improves to Grade 1 or less, the treatment may be restarted.
Conclusion
In conclusion, as blood involvement can occur in both early- and advanced-stage MF and SS, all patients should be assessed for such by flow cytometry, so that the disease stage can be accurately assigned, and patients managed accordingly.2 This is especially important, as studies have reported a correlation between blood involvement and both negative prognostic consequences and increased risk of disease progression, as well as placing it as intrinsic to the early pathogenesis of MF.5,7,13
There are a number of potential systemic therapeutic options that can be added to local, skin-directed, treatment,16,17 and studies investigating rational combinations of these are likely to be of high importance in the management of MF and SS.







