Interviewees: Grzegorz S. Nowakowski,1 Wojciech Jurczak2
1. Mayo Clinic, Rochester, Minnesota, USA
2. Maria Sklodowska-Curie National Research Institute of Oncology, Krakow, Poland
Disclosure: Prof Nowakowski has received consulting or advisory fees from Celgene/Bristol Myers Squibb, MorphoSys, Genentech/Roche, Selvita/Ryvu, Debiopharm, Kite/Gilead, and Karyopharm Therapeutics; and has received research funding from Celgene/Bristol Myers Squibb, NanoString Technologies, MorphoSys, and Genentech/Roche. Prof Jurczak has received advisory board and research funding from Debiopharm; and has received research funding from MorphoSys, Servier, Roche, and Celgene.
Acknowledgements: Medical writing was provided by Bronwyn Boyes, London, UK.
Disclaimer: The opinions expressed in this article belong solely to the two named interviewees.
Support: The publication of this interview feature was supported and reviewed by Debiopharm.
Citation: EMJ. 2021;6[1]:33-39.
Interview Summary
Diffuse large B-cell lymphoma (DLBCL) is an aggressive lymphoma and the most frequent non-Hodgkin lymphoma (NHL) in adults, accounting for 30–40% of cases. The standard of care (SOC) in first-line treatment of patients with DLBCL is the CD20 antibody rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP). Despite improvements in response and survival with R-CHOP chemotherapy, approximately 40% of patients with DLBCL will eventually relapse and 10% are resistant to first-line treatment (primary refractory).1 High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) is a salvage option for relapsed or refractory DLBCL.2 For patients in whom first-line treatment fails, and even more so for those who are not candidates for ASCT, prognosis is poor and the majority will succumb to the disease.3,4 Outcomes in refractory DLBCL have demonstrated that the survival from the start of salvage therapy was consistently poor, with a median overall survival (OS) of 6.3 months from the start of therapy.3 Therefore, an unmet need exists for more effective therapies in the second- and third-line settings.5 Furthermore, most patients with DLBCL are diagnosed at 60 years of age or older. Challenges to optimal therapy among older individuals include unfavourable biological features of DLBCL, geriatric vulnerabilities, suboptimal treatment selection, and low tolerance to aggressive chemotherapy toxicities.6,7 Several emerging therapies have demonstrated favourable efficacy and toxicity profiles compared with historical chemotherapy regimens, thus facilitating effective treatment for elderly patients in the relapsed or refractory setting. A novel approach to targeted treatment is antibody–drug conjugates (ADC). The major therapeutic advantage of ADC is their ability to selectively deliver a potent cytotoxic agent to target cancer cells, thereby minimising off-target effects.2 Novel targeted therapies that offer a chance for complete and durable response, and with an improved tolerability profile compared with current SOC, may reset expectations for DLBCL therapy.
UNMET NEED IN DIFFUSE LARGE B-CELL LYMPHOMA
Professor Grzegorz S. Nowakowski
DLBCL is the most common type of NHL. The prevalence of DLBCL in NHL cases ranges from 30% to 58% in Europe, and 25% to 35% in the USA.8 The disease is aggressive and patients typically present with rapidly enlarging lymphadenopathy, extranodal involvement, and constitutional symptoms, necessitating immediate treatment.9 Although DLBCL can affect children and young adults, it is most commonly diagnosed in those aged between 65 and 74 years, with a median age of 66 years at diagnosis.10,11 The SOC for first-line treatment is chemoimmunotherapy with R-CHOP, which leads to cure in approximately 60% of patients. Unfortunately, for the 40% of patients with refractory disease or who relapse (R/R), outcomes are particularly poor.12,13 Most relapses occur early on within 1–2 years of remission and only 10–15% will achieve cure with additional therapy.14,15
Positron emission tomography combined with CT (PET-CT) is now a routine part of staging DLBCL.16 This, coupled with physical examination and bloodwork, are the primary methods for monitoring patients and quantifying prognosis.17 Stratification of patients with DLBCL can be done on a clinical and molecular basis. Highly valuable clinical prognostic predictors in patients with DLBCL include the variables of the International Prognostic Index (IPI); patients with a high IPI (typically 3–5) have poorer outcomes.9,18 There are two major biologically distinct molecular subtypes of DLBCL: germinal centre B-cell (GCB) and activated B-cell (ABC). ABC DLBCL is associated with substantially worse outcomes when treated with standard chemoimmunotherapy. In addition to GCB and ABC subtypes, ‘double-hit’ lymphomas (DHL; approximately 5–10% of patients) and double-expressor lymphomas, which overexpress MYC and protein BCL2 (or less likely, BCL6), are aggressive DLBCL and are also associated with a poor prognosis.19 Triple-hit lymphoma is a rarer subtype of B-cell NHL where rearrangements are present in all three genes (MYC, BCL2, and BCL6).20 Unfortunately, none of these molecular classifiers currently impact the selection of therapy for patients, with the possible exception of DHL, which can occasionally be treated with more aggressive therapy in younger patients.21 For most other patients, regardless of clinical risk features and molecular features, the standard treatment is R-CHOP.
For patients with R/R disease, salvage therapy with high-dose chemotherapy and ASCT may offer a chance for a cure, but several factors may limit the utility of this approach. Patients who are older or have comorbidities may be inappropriate candidates for this approach, and patients with disease that is unresponsive to second-line chemotherapy may have poorer rates of long-term survival and incur added toxicity from the chemotherapy. To proceed to ASCT, patients require a response to the salvage therapy to begin with. Unfortunately, only approximately 50–60% of the R/R patients have a sufficient response to proceed to transplant.15 Even when including patients who undergo high-dose, salvage chemotherapy and subsequent ASCT, patients with R/R DLBCL have median 1-year and 5-year survival rates of 41% and 27%, respectively.9,22,23 Hence, in a search for improved outcomes in the R/R setting, clinical studies have focussed on DLBCL subtypes, especially in those ineligible for transplant or who have relapsed following transplant. An alternative option for patients in the relapsed setting is chimeric antigen receptor (CAR) T-cell therapy, which entails the genetic modification of autologous T cells via cloned DNA plasmids carrying a viral recombinant vector in addition to T-cell receptor-expressing genes. Unfortunately, as in ASCT, this is an intensive therapy, it is logistically complex, and it may not be available to all patients because of comorbidities and performance status, or rapidly progressive and symptomatic disease not allowing time for apheresis and CAR-T preparation, but also due to cost and limited access. Finally, not all patients will be willing and have enough social support to travel to treating centres to undergo this complex and time-intensive therapy.24
Unfortunately, many people still relapse after CAR-T therapy. Other recent entrants to the R/R DLBCL treatment landscape include the ADC polatuzumab vedotin-piiq, the selective inhibitor of nuclear export selinexor, and the anti-CD19 monoclonal antibody tafasitamab-cxix.25 Unfortunately, even with these novel agents, there remains a huge unmet need in this space. The majority of patients will relapse or progress after a period of time on these agents, and this can occur relatively early if they are nonresponders. There are also unique challenges in the third-line treatment of these patients. Firstly, tolerance to chemotherapy decreases with further lines of therapy. Furthermore, the ability to tolerate full-dose chemotherapy is also dependent on comorbidities, age, and performance status. Therefore, the doses of chemotherapy often require reduction in this patient population, which ultimately reduces the effectiveness,26 and the outcomes in older patients are generally worse than in younger patients. The other reason for possible worse outcomes is biology; for example, DHL and ABC-DLBCL are associated with worse outcomes, and these occur more commonly in elderly patients. Then there is the issue of comorbidities; for example, renal and kidney dysfunction often require an adjustment of the chemotherapy doses, thereby further reducing efficacy.27 Lastly, there are logistical issues for attending centres of excellence that can deliver the more complicated treatments, such as CAR T-cell therapy. This is especially true for the elderly and those who may live in rural settings. There is also a significant wait time and evaluation of those patients.24 Many of these patients will have rapidly progressive disease so they may progress in the time it requires to appropriately evaluate these patients and manufacture the CAR T-cell therapy, to the point that therapy cannot be used. It is important to note that in the past, subsequent lines of therapy were not highly effective and there may be a mindset, especially in older patients who have tried a first-line chemotherapy that did not work, that they are not willing to give it another try. Hopefully, this will change because of more effective and better-tolerated second-line treatment options, with many more in development.
There are now several approved therapies and many more in development, which will be approved shortly. There are several CD19 therapies available and in development, including naked antibodies, conjugated antibodies, bispecific antibodies, and CAR T-cell therapy,28 so targeting CD19 is clonally successful but has become a crowded space. A better understanding of the biology and of CD19 is needed before deciding how these therapies can impact CD19 expression and resistance to next-line therapy still targeting CD19. With an increasing number of treatment options, the next challenge will be ‘how to sequence treatment?’ This is where the art of medicine takes precedence, as it really depends on the patient situation, preferences, performance status, comorbidities, and toxicities from the previous treatment. In this context, alternative targets, such as CD37, are of huge interest: a novel target, potentially avoiding resistance to CD19-targeting compounds.
CD37 THERAPY FOR DIFFUSE LARGE B-CELL LYMPHOMA
Professor Wojciech Jurczak
The treatment spectrum for DLBCL has expanded significantly in recent years, particularly for patients with R/R disease. Mechanisms of action differ greatly among agents, reflecting the complex pathophysiology and genetic variations of the disease.24 The SOC for patients with R/R disease is salvage therapy with salvage chemotherapy (typically platinum-based), with ASCT consolidation in responding cases.15 Alternative approaches for those ineligible for ASCT include gemcitabine-based combination regimens, polatuzumab vedotin with bendamustine/rituximab (objective response rate [ORR]: 45%; median OS: 12.4 months; median duration of response: 10.3 months),29 and tafasitamab with lenalidomide (ORR: 58.8%; complete response [CR] rate: 41.3%; median duration of response: >24 months).30-33 Patients failing two lines of therapy may also be treated by selinexor (ORR: 28.3%; CR rate: 13%)34,35 or pixantrone-based regimens.36,37 CAR T-cell therapy plays an important role in the relapsed/refractory DLBCL setting, with reported 2-year remissions and a CR rate of 40% of patients (25% in DHL subtype).1
ADC are emerging as highly potent treatment options, combining chemotherapy and immunotherapy. This approach comprises a monoclonal antibody conjugated to the cytotoxic payload via a chemical linker that is directed toward a target antigen expressed on the cancer cell surface, reducing systemic exposure and therefore toxicity. The benefit of this group of agents is the ability to combine treatment modalities safely and effectively. In fact, the addition of a CD79b-targeted ADC, polatuzumab vedotin piiq, to bendamustine and rituximab more than doubled OS in patients with R/R DLBCL. The combination produced a CR rate of 40% and a median progression-free survival of 9.5 months.29,38
Other drugs in development include CD37-targeted agents. CD37 (tetraspanin TSPAN26) is a B-cell surface antigen widely expressed on mature B cells. CD37 is involved in immune regulation and tumour suppression. CD37 forms complexes with other tetraspanins and major histocompatibility complex (MHC) Class II antigens on B cells. CD37 is also important for T-cell–B-cell interaction, IgG/IgA production, and a balance between immune responses and tolerance.37 The loss of CD37 results in increased IL-6 signalling and STAT3 activation, which are both known to be involved in the pathogenesis of haematological malignancies.39 Oostindie et al.40 demonstrated that the combinations of hexamerisation-enhanced CD20 and CD37 antibodies co-operated in C1q binding and induced superior and synergistic complement-dependent cytotoxicity in patient-derived cancer cells, compared with the single agents. Consistent with these data, a strategy of dual-ligand immunoliposomes of anti-CD20 combined with anti-CD37 demonstrated highly specific targeting to both leukaemia cell lines and B-cell chronic lymphocytic leukaemia patient cells. Compared with the single-antibody immunoliposomes, the combination demonstrated superior delivery efficiency and apoptosis induction to B-cell chronic lymphocytic leukaemia patient cells. These findings provide novel insights into the mechanisms of synergy in antibody-mediated, complement-dependent cytotoxicity, provide a rationale to enhance the co-operativity and therapeutic efficacy of antibody combinations, and provide a preferred strategy of personalised nanomedicine for the treatment of B-cell malignancies.41
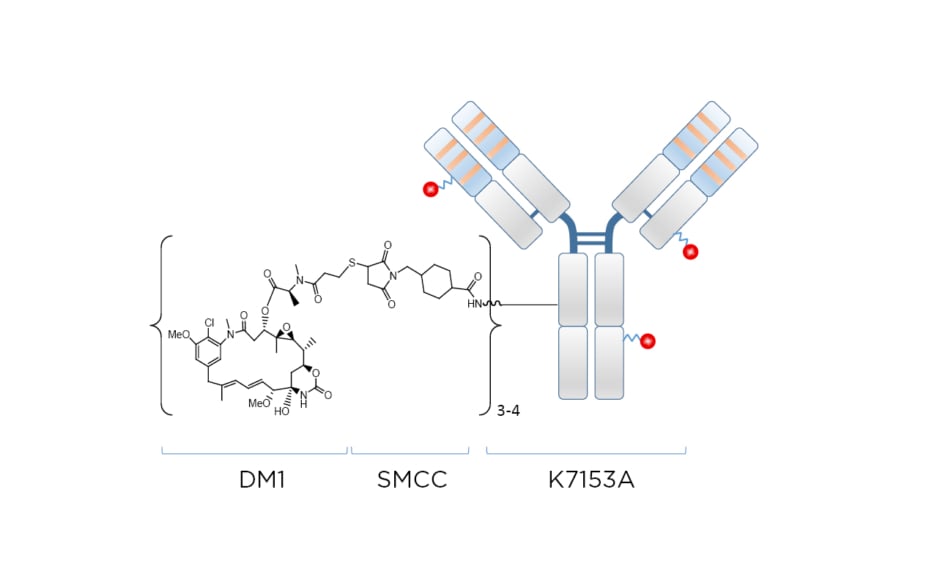
Naratuximab emtansine (IMGN529 or Debio 1562 [Debiopharm, Lausanne, Switzerland]) is an investigational ADC comprising a CD37-targeting antibody conjugated to the maitansine-derived microtubule disruptor DM1 via a succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) linker, forming a nonreducible thioether bond (Figure 1). The CD37 antigen is widely present on the surface of cancerous blood cells in NHL, but absent on normal stem cells and plasma cells. Targeting the CD37 antigen on blood cancer cell surfaces can help to promote cancer cell death by specifically delivering the cytotoxic payload DM1. Naratuximab emtansine binds with high affinity and specificity to CD37, obstructing cell proliferation pathways while allowing internalisation, processing, and intracellular release of the DM1 payload. As a result of its ability to disrupt microtubule assembly, DM1 subsequently induces cell cycle arrest and apoptosis.42
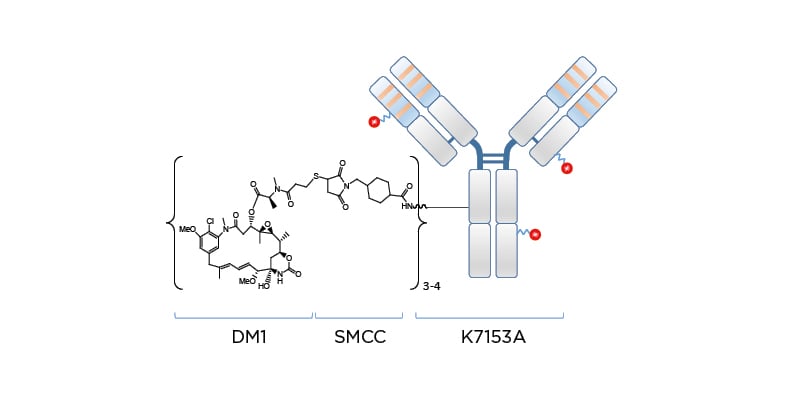
Figure 1: Naratuximab emtansine: an innovative antibody–drug conjugate targeting CD37.
DM1: microtubule disruptor; SMCC: succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate.
A first-in-human, Phase I trial in 49 adult patients with R/R B-cell NHL found that the most frequent treatment-emergent adverse events (AE) over all dose levels tested were fatigue (39%), neutropenia (37%), pyrexia (37%), and thrombocytopenia (37%). AE that led to treatment discontinuation occurred in 10 patients (20%). Eight patients (16%) had treatment-related serious AE, the most common being Grade 3 febrile neutropenia. Five (13%) of 39 response-evaluable patients achieved an overall response (one CR and four partial responses), four of which occurred in the subgroup of patients with DLBCL (Table 1).43 A preclinical study found that the combination of naratuximab emtansine and rituximab was more potent than either agent alone, and the benefit of this combination was associated with increased apoptotic induction and cell death.44
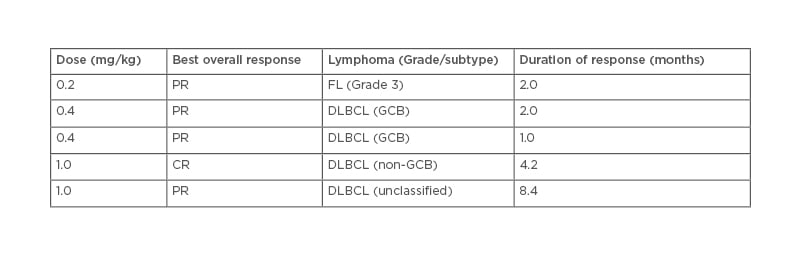
Table 1: Disease subtypes and duration of response for patients with an objective tumour response.
First-in-human, Phase I trial assessing the safety and preliminary activity of naratuximab emtansine in adult patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. The primary objective was to determine the maximum tolerated dose and recommended Phase II dose.43
CR: complete response; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; GCB: germinal centre B cell; PR: partial response.
These findings, taken in combination with additional studies showing that the combination of naratuximab emtansine and rituximab was highly efficacious in multiple xenograft models, suggest a novel mechanism whereby the potency of naratuximab emtansine can be enhanced by CD20 binding, which results in the increased internalisation and degradation of naratuximab emtansine leading to the generation of greater amounts of cytotoxic catabolite.39-45 Overall, these data provide a biological rationale for the enhanced activity of naratuximab emtansine in combination with rituximab, and support the ongoing Phase II46 clinical evaluation of naratuximab emtansine in combination with rituximab in patients with R/R DLBCL. The objective of this Phase II study of 100 patients is to establish the safety and efficacy profile using two different administration regimens. Results of this Phase II study are promising, with durable responses and mild adverse events; further results will be released in due course. Naratuximab emtansine in combination with rituximab may be a very promising treatment option and could become a SOC for a subpopulation, including the elderly, for patients with relapsed/refractory DLBCL. The very favourable toxicity profile of naratuximab emtansine allows treatment of patients with severe comorbidities, where few other therapeutic options are available.
In conclusion, the promising data from recent trials highlight the importance of understanding the unique prognoses and responses that DLBCL subtypes confer on patient outcomes. The establishment of DLBCL subtypes as prognostic and therapeutic response factors will further fuel a search for more specific molecular targets in the disease process. In addition, new therapeutic options with distinct mechanisms of action are needed to address the unmet medical need, which still exists for DLBCL, particularly in the first-line treatment of patients who are elderly or frail, and in the relapse/refractory setting for patients who are unfit for ASCT or who relapse afterwards.