Presenters: Mark Reding,1 Maria Elisa Mancuso,2 Gili Kenet,3 Pål Andrè Holme,4 Katharine Batt5
1. Center for Bleeding and Clotting Disorders, University of Minnesota Medical Center, Minneapolis, Minnesota, USA
2. Center for Thrombosis and Haemorrhagic Disease, Humanitas Clinical and Research Center – IRCCS, Milan, Italy
3. Israel National Hemophilia Center and Thrombosis Institute, Chaim Sheba Medical Center, Ramat Gan, Israel
4. Department of Haematology, Oslo University Hospital and Institute of Clinical Medicine, University of Oslo, Oslo, Norway
5. Wake Forest Baptist Medical Center, Winston-Salem, North Carolina, USA
Disclosure: Dr Reding has received funding from Bayer, BioMarin; consultant fees from Bayer, CSL Behring, Novo Nordisk, Sanofi Genzyme, and Takeda; speaker bureau fees from Bayer, CSL Behring, Sanofi Genzyme, and Takeda. Dr Mancuso has received consultant/speaker bureau fees from Bayer, Biotest, Bioverativ, Catalyst, CSL Behring, Grifols, Kedrion, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, and Sobi. Dr Kenet has received grant/research support from Bayer, Pfizer, Roche, Sanofi, PI Healthcare, and CSL Behring; consultant fees from Bayer, Pfizer, BioMarin, Takeda, Roche, Novo Nordisk, and Sanofi; speaker bureau fees from Bayer, Pfizer, Takeda, BioMarin, and Novo Nordisk. Dr Holme has received grant/research support from Bayer, Octapharma, Pfizer, and Shire; consultant fees from Bayer, Novo Nordisk, Octapharma, Pfizer, Shire, and Sobi. Dr Batt acted as a consultant to Bayer in the interpretation of data for this manuscript. Dr Batt is also a scientific advisor to the health consulting firm, Precision Health Economics.
Acknowledgements: Medical writing was provided by Bronwyn Boyes, London, UK.
Support: This publication was supported and reviewed by Bayer.
Citation: EMJ Hematol. 2021;9[Suppl 4]:2-9.
Approval Number: MA-M_DAM-ALL-0334-1
Meeting Summary
At the 14th Annual Congress of the European Association for Haemophilia and Allied Disorders (EAHAD), held virtually, data on the efficacy, safety, pharmacokinetics, and pharmacoeconomic s of BAY 94-9027 (damoctocog alfa pegol; Jivi®) in severe haemophilia were presented across five posters, and further demonstrate the positive clinical profile of BAY 94-9027 in clinical trials.BAY 94-9027 is a recombinant factor VIII (rFVIII) product, site-specifically PEGylated with a 60 kDa polyethylene glycol (PEG) to reduce clearance and extend time in circulation.1-4 It is approved for use for prophylaxis and the treatment of bleeds in patients with haemophilia A aged ≥12 years in several countries including the USA, the European Union (EU), Japan, and Canada.5-8
The efficacy and safety of BAY 94-9027 for the prevention and treatment of bleeds was demonstrated in the multinational, partially randomised, Phase II/III PROTECT VIII study9 (NCT01580293) in adolescent and adult male patients with severe haemophilia A10 and independently evaluated in a paediatric male population in the PROTECT VIII Kids study11 (NCT01775618) of previously treated patients aged <12 years with severe haemophilia A.12 Following completion of PROTECT VIII and PROTECT VIII Kids, patients could continue to receive BAY 94-9027 in the now completed, open-label extension studies that evaluated safety and efficacy for 100 or more exposure days.13,14 To complement these long-term safety and efficacy commitments, a post-marketing interventional study is underway in Europe.15 All studies enrolled male subjects only as haemophilia predominantly affects males.16
At EAHAD 2021, post hoc analyses from the PROTECT VIII and PROTECT VIII Kids extension trials were presented in two posters, showing that the efficacy and safety of BAY 94-9027 has been maintained for a median observation period of 6 years and more. In a third poster, the effective bleeding control reported for BAY 94-9027 prophylaxis regimens used in PROTECT VIII was accompanied by mean FVIII trough levels above 1% for all regimens, suggesting that patients with haemophilia A can be treated effectively with different regimens based on the clinical bleeding phenotype. A snapshot analysis of the post-marketing interventional study presented in a fourth poster showed that the favourable safety and efficacy profile of BAY 94-9027 prophylaxis reported in PROTECT VIII and its extension is also observed in routine clinical use. Lastly, as it may be difficult for clinicians to distinguish between rFVIII products with the lack of head-to-head randomised clinical trials (RCT),17,18 a matched-adjusted indirect comparison (MAIC) analysis was presented. These results showed that BAY 94-9027 was associated with significantly lower consumption and ≥20% reduction in annual rFVIII use than other products (namely efmoroctocog alfa, rurioctocog alfa pegol, and turoctocog alfa pegol).17
Efficacy and Safety of BAY 94-9027 Prophylaxis Is Sustained for ≥6 Years: Outcomes in 22 Patients in the PROTECT VIII Extension Study
This presentation reported bleeding and safety outcomes of the patients who received at least 6 years prophylactic treatment with BAY 94-9027 during the PROTECT VIII main study and its extension.14,19,20
Twenty-two adults and adolescent patients with severe haemophilia A were treated with BAY 94-9027 prophylaxis for at least 6 years. This consisted of either 30–40 IU/kg twice a week (n=2), 45–60 IU/kg every 5 days (n=4), or 60 IU/kg every 7 days (n=5); those patients who switched between regimens were considered as a variable frequency (VAR) group (n=11).19,20 The median age of patients was 43.5 years (range: 12−59 years), median weight was 92.1 kg (59−119 kg). Eighteen (81.8%) patients received prophylaxis 12 months prior to the study. The median time in the extension was 5.6 years (range 5.3−6.3 years), with a median FVIII consumption of 3414 IU/kg per year.20 At the end of the extension, most patients were treated with regimens of every 5 days (n=11) or every 7 days (n=6).19
For the 22 patients with ≥6 years of BAY 94-9027 prophylaxis, the median total and joint annualised bleeding rates (ABR) decreased throughout the main study and extension. As can be seen in Figure 1, the median (quartile [Q]1; Q3) ABR for the full extension period was 0.9 (0.4; 2.2) compared with a pre-study ABR of 3.0 (1.0; 15.0). The median joint ABR was also reduced (0.6 [0.4; 1.7]) compared with a pre-study joint ABR of 3.0 (0.0; 12.0).19,20 Over the entire extension period, 22.7% of patients had 0 total or joint bleeds with 72.7% having 0 total or joint bleeds during the last 6 months of the extension period.19,20
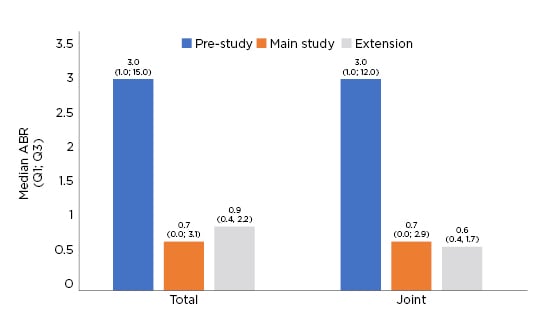
Figure 1: Total and joint annualised bleeding rate.
Q: quartile.
Safety data showed that drug-related adverse events (AE) were reported in four of the 22 patients (18.2%) and were either mild (n=2; elevated alanine aminotransferase, arthralgia) or moderate (n=2; bone marrow oedema and meniscal degeneration in one patient, osteoarthritis in another patient). None of the patients experienced a study drug-related serious AE, thrombotic, or fatal event and none developed FVIII inhibitors.19,20
In summary, treatment with BAY 94-9027 as prophylaxis in patients with severe haemophilia A maintained efficacy for 6 years and longer, with median total and joint ABR <1 during the PROTECT VIII extension. No unexpected drug-related AE or changes in the safety profile over time were reported during the extension. Prophylactic treatment with BAY 94-9027 was efficacious and well tolerated over a long period of time. These data may support the long-term use of BAY 94-9027 prophylaxis in patients with haemophilia A.19,20
BAY 94-9027 Prophylaxis Sustains FVIII Trough Levels Above 1% in all Treatment Regimens in Patients with Severe Haemophilia A from PROTECT VIII
Maria Elisa Mancuso
PROTECT VIII, the pivotal Phase II/III study, demonstrated the efficacy and safety of prophylactic BAY 94-9027 in previously treated, severe haemophilia A patients (FVIII <1%) aged 12–65 years.10 The design of PROTECT VIII was based on patient bleeding phenotype, and chosen to reflect the real-world clinical approach to prophylaxis in which dosing regimens are tailored with the aim to optimise bleed control while using infusion schedules that best fit the individual needs and preferences of patients.10 While patient bleeding phenotype, rather than pharmacokinetics, was used to inform the treatment regimen used for each patient in PROTECT VIII, it was of interest to determine what BAY 94-9027 FVIII trough levels accompanied the effective bleeding control reported during PROTECT VIII and its extension.21,22
Trough levels were determined by chromogenic assay at Weeks 20 and 36 in the main study and every 6 months in the extension.21,22 Only trough levels within a post-infusion interval corresponding to the assigned regimen were included in the analysis. The time since last injection prior to sampling was calculated based on electronic patient diary records of prophylactic infusions.22
The results showed that all patients who received BAY 94-9027 in the PROTECT VIII study and its extension achieved mean FVIII trough levels above 1%, including those treated with the once-weekly regimen (Figure 2).22
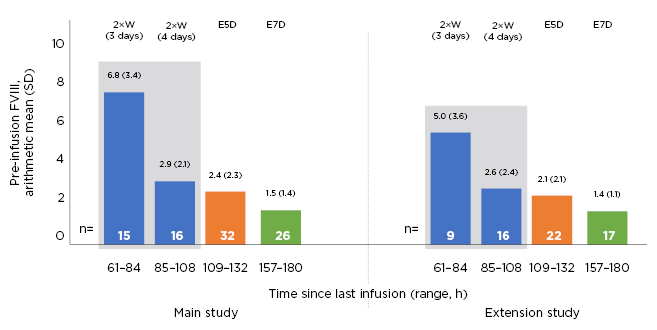
Figure 2: Pre-infusion (trough) factor VIII levels in PROTECT VIII main and extension studies in patients with prophylaxis.
E5D: every 5 days; E7D: every 7 days; FVIII: factor VIII; SD: standard deviation; 2×W: twice weekly.
During the main study, the pre-injection arithmetic mean (standard deviation, SD) FVIII trough levels in patients treated with prophylaxis were 6.8 (3.4) and 2.9 (2.1) IU/dL for those treated, with 3 (n=15) and 4 (n=16) days since last injection, and 2.4 (2.3) and 1.5 (1.4) IU/dL for every 5 days (n=32) and every 7 days (n=26), respectively. During the extension, the corresponding values for those treated twice weekly, with 3 (n=9) and 4 (n=16) days since last injection, every 5 days (n=22) and every 7 days (n=17) were 5.0 (3.6), 2.6 (2.4), 2.1 (2.1), and 1.4 (1.1) IU/dL, respectively.21
To summarise, average FVIII trough levels were sustained above 1 IU/dL in patients who received BAY 94-9027 prophylaxis during the PROTECT VIII study with all treatment regimens. These data support the efficacy of personalised BAY 94-9027 treatments using individualised infusion schedules that best fit the needs of the patient. They also suggest that patients can be treated effectively with different regimens based on the clinical bleeding phenotype.21,22
BAY 94-9027 Provided Effective Long-term Prophylaxis in Paediatric Patients aged ≥12 years at the End of the PROTECT VIII Kids Extension Study, Indicating Consistent Safety of Treatment into Adolescence
Gili Kenet
It is important that treatment regimens for paediatric and adolescent patients with haemophilia A can be individualised to address personal preferences and lifestyles.23 Data from the PROTECT VIII Kids study and its extension have shown that BAY 94-9027 may be efficacious for the prevention and treatment of bleeds in children <12 years with severe haemophilia A.12,13 This presentation reported on data from the PROTECT VIII Kids extension study24 which analysed bleed protection in a subgroup of previously treated patients aged ≥12 years at the end of the extension.
In the PROTECT VIII Kids study,12 patients aged <12 years with severe haemophilia A received BAY 94-9027 prophylaxis twice weekly (25−60 IU/kg), every 5 days (45−60 IU/kg), or every 7 days (60 IU/kg).12,13 The dose and dosing frequency could be changed at any time at physician discretion. Patients in the optional extension study included those completing ≥50 exposure days or ≥6 months in the main study or a 12-week safety sub-study of patients aged <6 years.13 The main efficacy outcome measured was ABR for total, joint, spontaneous, and traumatic bleeds. Safety assessments included measuring and monitoring frequency of inhibitor development; AE (including serious AE and study-drug-related AE); anti-BAY 94-9027 antibodies; anti-PEG antibodies; quantitative plasma PEG levels; renal biomarkers; weight and vital signs.13,25
Overall, 73 patients were enrolled in the main or expansion studies. Of these, 61 were eligible for and 59 entered the extension study, with 57 patients completing this period. At study enrolment, patients had a median age of 9 (range: 5−11) years, with 30 patients aged median 15 (range: 12–18) years at the end of the extension. In this group, median time in the study was 6.1 years (range: 2.5−6.6 years) overall and 5.5 years (range: 2.0−5.9 years) in the extension study.25
The most common dosing regimen at the end of the extension period was 25–60 IU/kg twice weekly for patients aged ≥12 years old (n=16), with an overall median dose per infusion of 45.4 (range 24−60) IU/kg.25 The cumulative number of BAY 94-9027 exposure days in the extension period was 430.0 (range: 210−612)25 with the median (Q1;Q3) number of infusions per year being 89.7 (73.6;104.3).24 The median total dose for the extension period was 3,945.8 (range 2,459−5,739) IU/kg/year.25
Overall, during the extension period, median (Q1;Q3) ABR was 1.8 (0.5;3.3)24,25 but decreased to 1.5 (0.0;4.0) in the last 12 months of treatment. ABR for spontaneous bleeds was generally low (0.4) and the majority of bleeds were joint bleeds (0.7) and traumatic bleeds (0.7). More than 85% of bleeds were mild-to-moderate in intensity.25
Three patients (10%) experienced a study drug-related AE, two of which were mild, and one was moderate in intensity. These AE included arthralgia (n=1) and development of suspected FVIII inhibitors (n=2); however, none of the suspected inhibitors were confirmed in a second sample. All renal biomarkers were consistent with normal ranges. Plasma PEG was detected at a single time point in two patients and at repeated timepoints in five patients. Levels were approximately at the detection limit (0.1 mg/L) and either remained the same or fell below the detection level over time. There were no anti-PEG antibodies detected,25 no reports of thrombotic events, and no deaths.24
In summary, BAY 94-9027 prophylaxis was efficacious (providing consistent levels of bleeding control) and well tolerated at all dosing regimens in paediatric patients with severe haemophilia A aged ≥12 years at the end of the extension study. These results were observed for a median time of over 6 years in the PROTECT VIII Kids main and extension studies, and support the continuous use of BAY 94-9027 for these patients into adolescence. In summary, these results may indicate that patients could safely continue BAY 94-9027 treatment into adolescence, providing continued improvement in bleeding protection in this young, active population.24,25
Safety and Efficacy of BAY 94-9027 Prophylaxis in Patients with Severe Haemophilia A: Interim Results of a Post-marketing Interventional Study
Pål Andrè Holme
This presentation reported interim analysis of safety and efficacy data from the ongoing multicentre, single group, uncontrolled, open-label, Phase IV,15 interventional post-marketing study of BAY 94-9027 prophylaxis in routine clinical use (NCT04085458) in previously FVIII-treated (≥150 exposure days) patients aged ≥18 years with severe haemophilia A (FVIII:C <1%).26
Eligible patients will receive BAY 94-9027 prophylaxis for 100 exposure days. The recommended starting dose is 45 IU/kg every 5 days or twice weekly until the next planned visit (Visit 3 after 8−10 weeks), with an option to continue or increase the dose or switch regimen to either twice weekly or every 7 days thereafter, based on patient needs and bleeding events. Patients who switch prophylaxis regimen after their third visit will be assigned to a VAR group.
The primary endpoint is FVIII inhibitor development (titre ≥0.6 Bethesda units) with secondary endpoints including treatment emergent AE, anti-PEG antibody development, and ABR. Bleeds and BAY 94-9027 treatments were recorded using electronic patient diaries.26,27
Patients who completed at least 24 weeks of treatment were included in this snapshot analysis.27 By 1st September 2020, 32 patients were enrolled and 18 had received BAY 94-9027 prophylaxis for ≥24 weeks (twice weekly, n=2; every 5 days, n=7; every 7 days, n=5; VAR, n=4).26,27
At data cut-off, the median (range) total time in the study was 235.2 (185–293) days with 48 (34–80) exposure days. Median total and joint ABR were <2.0 during the time in the study; one-half of the cohort had 0 total bleeds and two-thirds had 0 joint bleeds (Figure 3).27
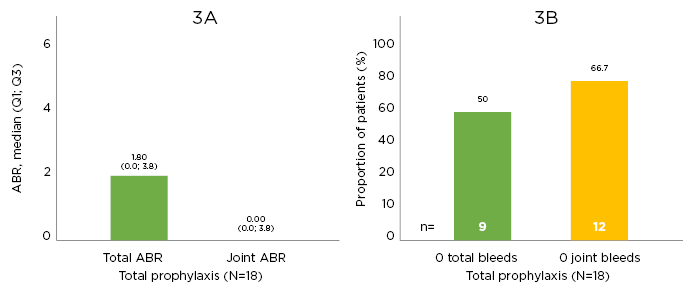
Figure 3: Efficacy outcomes.
Efficacy outcomes in A) annualised bleeding rate outcomes and B) zero bleed outcomes. ABR: annualised bleeding rate; Q: quartile.
Eight patients (44.4%) experienced an AE, six of which were mild, one was moderate, and one was severe in intensity. None of the reported AE were considered by the investigator to be related to the study drug. There were no anti-PEG antibodies detected and no patients developed FVIII inhibitors.26,27
In summary, preliminary analyses from 18 adult haemophilia A patients observed for ≥24 weeks show that individualised prophylaxis regimens of BAY 94-9027 were well tolerated in this analysis of post-marketing study data. No immunogenicity concerns were observed and none of the patients developed FVIII inhibitors or PEG or anti-drug antibodies. These data support the favourable safety and efficacy profile of BAY 94-9027 prophylaxis in routine clinical use.26,27
Indirect Comparison of Annualised Bleeding Rate and Annual Consumption Between Extended Half-Life Recombinant Factor VIII Products Shows Differences in Consumption with Similar Outcome
Katharine Batt
Extended half-life (EHL) rFVIII agents used to prevent bleeds in persons with haemophilia A include BAY 94-9027, efmoroctocog alfa, rurioctocog alfa pegol, and turoctocog alfa pegol. These agents have been investigated in single agent trials; however, no direct head-to-head RCT have been conducted to compare between agents to date.17,18,28 Therapeutic clinical data for rare diseases are often limited due to small patient populations and other ethical considerations for RCT,29 thereby limiting comparisons between therapies. This presentation reviewed an indirect comparison of EHL rFVIII products.17,28
The objective of this analysis was to compare bleeding rates and rFVIII consumption of prophylaxis regimens between EHL rFVIII treatments using MAIC.17,28 MAIC is a validated method for comparing outcomes of clinical trials in the absence of head-to-head comparisons.17,18,28 MAIC uses patient data from individual treatment trials to match baseline summary statistics (after adjusting for heterogeneity in baseline characteristics between populations). MAIC has previously been established as an effective tool for comparing outcomes of haemophilia therapies in the absence of RCT.18
Patient-level data from the PROTECT VIII study of BAY 94-9027 were weighted against aggregate data from A-LONG (efmoroctocog alfa),30 PROLONG-ATE (rurioctocog alfa pegol),31 and pathfinder 2 (Novo Nordisk, Bagsværd, Denmark; turoctocog alfa pegol)32 studies to match baseline characteristics including age, race, weight, prior treatment, region (efmoroctocog alfa only), and bleeding events in the prior year (efmoroctocog alfa and turoctocog alfa pegol). Outcomes from this analysis included ABR, percentage of patients with no bleeds, and annual rFVIII consumption.17,28
After matching, BAY 94-9027 had a similar mean ABR as the comparators with no significant difference between them: BAY 94-9027: 3.77 versus efmoroctocog alfa: 3.90; BAY 94-9027: 3.95 versus rurioctocog alfa pegol: 3.70; BAY 94-9027: 4.10 versus turoctocog alfa pegol: 3.70. BAY 94-9027 had a similar, nonsignificant, percentage of patients with 0 bleeds as the comparators: 34.1% versus 40.7% for efmoroctocog alfa; 38.9% versus 39.6% for rurioctocog alfa pegol; 41.0% versus 40.0% for turoctocog alfa pegol.17,28 Mean annual consumption for BAY 94-9027 was 20% lower than efmoroctocog alfa (3469.9 versus 4289.1 IU/kg/year, p<0.001) and 27% lower than turoctocog alfa pegol (3355.1 versus 4560.4 IU/kg/year, p=0.003). Median annual consumption for BAY 94-9027 was 26.7% lower than rurioctocog alfa pegol (3552.4 versus 4845.0 IU/kg/year; p values were incalculable due to unavailability of standard errors for median consumption).28
In summary, for patients with haemophilia receiving EHL rFVIII prophylaxis, BAY 94-9027 was associated with significantly lower consumption, with ≥20% reduction in annual rFVIII use while achieving similar ABRs and 0 bleed rates, compared with other EHL agents analysed.17,28

![EMJ Hematology 9 [Supplement 4] 2021 Feature Image](https://www.emjreviews.com/wp-content/uploads/2021/08/EMJ-Hematology-9-Supplement-4-2021-Feature-Image-940x563.jpg)





