Meeting Summary
The main objectives of this symposium were to review the value of biosimilars in sustainable treatment for haematologic malignancies and to recognise the developmental differences between biosimilars and their reference products. The meeting also aimed to evaluate the data on monoclonal antibodies for the treatment of haematologic malignancies and the role of biosimilars to address gaps in healthcare. Dr Cornes highlighted recent innovations in cancer treatment and presented biosimilars as economic tools that can address the financial issues that hamper progress. Prof Vulto discussed the need for healthcare professionals to be well informed about the principles of biosimilarity and aware of current and emerging therapies. Prof Jurczak presented the case for rituximab (and its biosimilars) as the standard of care for first-line B cell non-Hodgkin’s lymphoma (NHL) and its potential as maintenance treatment for indolent NHL (iNHL).
The Role of Biosimilars in Promoting Sustainability of Care
Doctor Paul Cornes
Advances in Oncology Therapy
Dr Cornes began his presentation by stating that the field of oncology is going through a transformation. Anti-cancer medicines are the tools that power the advances in oncology and haematology; since 1975, it is estimated that new therapies have accounted for 50–60% of the increase in cancer survival rates.1 The past five decades have seen a remarkable increase in the rate of innovation in cancer drugs. Twenty-three new drugs were introduced in the 2000s and 51 between 2010 and 2015.2-4 More than 800 cancer therapies were in development in 20155 and, if this rate continues, this decade could add >100 new cancer drugs by 2020. These developments, which are transforming cancer care, reflect new targeted precision medicines. In some cancers, such as chronic myeloid leukaemia, personalised models of therapy have tripled survival rates from 6 years to >22 years.6 While this rate of innovation has the potential to offer more choices for patients and healthcare professionals, it can create financial problems for hospital pharmacists sourcing these medications.
Economic Issues and the Affordability of New Therapies
Despite major advances in innovative therapies, a key issue hindering improvement in outcomes of cancer management year-on-year is affordability. It has been reported that, from a selection of countries studied, patients in only three countries, USA, France, and UK, had access to routine reimbursement for at least half of oncology medicines launched in 2014 and 2015.7,8 The current situation is summarised by Thomas et al.9 in their report from the WISH Delivering Affordable Cancer Care Forum 2015: “We must confront a stark reality: cancer care is not affordable for most patients, many payers, and nearly all governments. This is a real and immediate issue around the world.” This sentiment was reflected in an audience poll during the symposium, where the majority of delegates agreed that access to reimbursement of biologic therapies for their patients was a moderate-to-significant problem.
European Union Strategies to Overcome the Funding Gap: The Use of Biosimilars
It has become clear that funding for drug development will have to come from savings within current budgets, while ensuring these savings do not compromise patient care. In 2016, the European Union (EU) published the Joint Report on Health Care and Long-Term Care Systems and Fiscal Sustainability, which recommended: “Policies should strengthen the cost-effective use and the affordability of medicines, by promoting public procurement and the role of generics and biosimilars.”10 The IMS Institute for Healthcare Informatics reported that the cumulative potential savings to health systems in the five major EU markets and the USA, as a result of the use of biosimilars, could exceed €50 billion in aggregate over the next 5 years.11
Biosimilars are copies of patent-expired biologic medicines approved by a ‘biosimilar’ regulatory pathway. They have the same indications, quality, safety, and efficacy of the original reference medicine, and in Europe they share the same international non-proprietary name as their reference product. They have been in use in Europe since 2006, with no evidence to date that they perform any differently than the original reference drugs.12 More than 30 biosimilars have been used over the past 10 years in Europe, accounting for >400 million patient-days’ exposure.
High-cost biologic targeted therapies are associated with economic challenges that cheaper, equally effective, biosimilars could resolve. Currently, due to funding restraints, effective therapies are held back for later stages of disease, and are reserved for only the most severe cases, although indications are often much wider. Many innovative therapies are unaffordable and budgets for certain therapy areas are inadequate. Biosimilars can offer solutions to these problems. They can be used in patients at earlier stages of disease, and create savings that can be used to access previously unaffordable therapies or for other areas of unmet need.13 Ten years of biosimilar experience in Europe has proven that these cost-saving effects are achievable, demonstrated by the following examples.
A granulocyte colony stimulating factor (G-CSF), filgrastim, is used to prevent neutropenia during chemotherapy. In London, during the first 2 years of biosimilar filgrastim use, five-times more patients were treated, generating savings of almost €3 million/year. In addition, the use of biosimilar filgrastim enabled treatment to be given to lower-risk patients and those at an earlier stage of disease.14 A similar impact of biosimilar filgrastim was reported in Sweden, which experienced a five-fold increase in daily granulocyte colony stimulating factor usage, and net savings of €2 million, representing 4–5% of their total drug budget.15 In New Zealand, the biosimilar filgrastim was introduced in 2012 and generated savings of $5 million/year.16 Prior to biosimilar filgrastim use, approximately one-third of women receiving docetaxel-based chemotherapy suffered from neutropenic fever and required admission to hospital. By 2014, this figure was reduced to <7%.17
Across Europe, it has been demonstrated that biosimilars bring treatments into reimbursement that might otherwise be unaffordable (Figure 1).
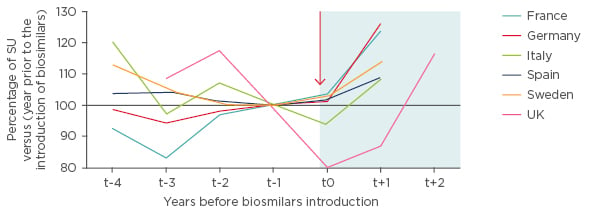
Figure 1: Trends in use of white-cell growth factors (G-CSF) before and after biosimilar introduction in the European Union.18
G-CSF: granulocyte colony stimulating factor; SU: standard units.
Drug reimbursement decisions are often made using health technology assessments with cost-effectiveness criteria. In the UK and Netherlands, biosimilars have been shown to reverse negative reimbursement decisions. In 2008, the National Institute for Health and Care Excellence (NICE) decided that although epoetins were clinically effective in correcting chemotherapy-induced anaemia, they were not cost effective at list price; however, by 2014, biosimilar price competition prompted a cycle of price reductions and led to a reduction in epoetin contract prices. The NICE accepted this change and reversed their previous decision, meaning these products are now used routinely in our health service.19
The promise of biosimilars is evidenced by every measure of economic success. Innovative pharmaceutical manufacturers have outlined plans to adapt to biosimilars, noting that, although they may lose sales from patent-expired medicines, savings will allow payers to reinvest in their next generation of innovation (Figure 2).
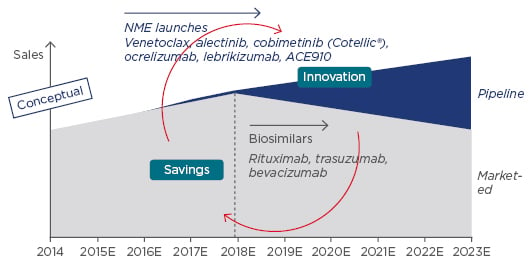
Figure 2: Biosimilars are expected to affect sales in coming years.
NME: new molecular entity.
Adapted from Lorenzetti.20
As of June 2017, many versions of rituximab have European approval. Roche, Sandoz, and Celltrion market their products Mabthera™, Rixathon™, and TruximaTM, respectively, across multiple indications: follicular lymphoma, diffuse B-cell lymphoma, chronic lymphocytic leukaemia (CLL), rheumatoid arthritis, and granulomatosis with polyangiitis and microscopic polyangiitis.21-23 Roche’s Mabthera was the original reference product and gained approval through pivotal Phase III trials in each indication. The biosimilars Rixathon and Truxima, however, gained approval from equivalence clinical trials in one of these many indications. By proving their comparability with the reference product, their approval could be extrapolated across many indications.
Patent laws in Europe give the reference drug manufacturer a period of monopoly sales; upon patent-expiry, other manufacturers are free to create biosimilar versions of the reference drug. Although drugs are approved at a European level, each country served by the European Medicines Agency (EMA) has a separate legal system, and not all patents expire at the same time in every country. This has given rise to ‘bio-identicals’, versions of the same drug sold under different brand names, used in different territories due to variations in patent expiry dates.24 For this reason, physicians and pharmacists will need to know that Europeanapproved rituximab Rixathon and rituximab Truxima will be available in certain regions with different brand names.
The World Health Organization (WHO)’s essential medicines list outlines the minimum medicine needs for a basic healthcare system, listing the most efficacious, safe, and cost-effective medicines for priority conditions. The WHO defines medicine use as ‘rational’ when patients receive appropriate medicines, in doses that meet their own individual requirements, for an adequate period of time. A fourth step has been added to these criteria, stating that medicines should be used at the lowest cost, both to the patient and the community.25 This adds weight to the payers’ argument for therapeutic biosimilar oncology products that could replace the three biologics (filgrastim, trastuzumab, and rituximab) on the latest WHO essential medicines list for cancer.26
It is important to understand the barriers to biosimilar use in order to overcome them. A Belgian study, which surveyed physicians, pharmacists, payers, and industry experts, reported that the key obstacles to biosimilar use are lack of confidence, uncertainty about interchangeability, and lack of financial incentive.27 Strategies to overcome these issues in Belgium included the introduction of country-wide educational programmes and minimum-use quotas. While each national health system will have unique issues to consider when introducing biosimilars, studies across 23 countries by the Harvard Business School confirm that multi-stakeholder policy planning is crucial. As well as drug pricing, parallel programmes such as patient and physician education coupled with non-financial incentives (e.g. simplified paperwork for physicians using biosimilars) have played a significant role in shaping biosimilar uptake.28,29
A Look at Biosimilar Development
Professor Arnold G. Vulto
Biosimilars and Bio-questionables: Prescribing Criteria and Uncertainty Issues
Prof Vulto began by emphasising that biosimilars are products that have been licensed by the EMA/US Food and Drug Administration (FDA) or according to WHO regulatory pathways. These regulatory pathways require similarity to be proven in an extensive comparability exercise, encompassing physicochemical, biological, and pharmacological properties; they do not aim to prove efficacy, but to assess similarity. Products which do not meet ‘similarity’ standards are known as bio-questionables; they have not been endorsed by the necessary regulatory bodies and should not be mistaken for biosimilars. There is concern that reports of poor efficacy and immunogenicity of bio-questionables may taint the reputation of biosimilars. However, based on the European Commission report, over the past 10 years there have not been any safety incidents with any EMA-approved licensed biosimilars in Europe,15 which should provide reassurance to clinicians considering their use.
A set of criteria must be satisfied for a physician to prescribe a biosimilar product and a pharmacist to dispense it. It is important that the physician has sufficient confidence in the ‘sameness’ of the biosimilar to the reference product, and that, in the case of transitioning from an originator to a biosimilar, patients trust that the product has the same action and safety as their previous therapy. Talking to patients to reassure them about the reasons for switching to a different, cheaper drug reduces the risk of the ‘nocebo’ effect. The pharmacist must be allowed to dispense the biosimilar and must have sufficient incentive to do so.
There is a very different focus on the development of biosimilars, compared to their original reference products. While the major goal of reference medicine trials is to determine the clinical effect (i.e. efficacy and safety in patients), biosimilar trials aim to determine similarity, and therefore establish a scientific bridge to the clinical efficacy of the reference. Ultimately, both approaches aim to provide the same level of confidence with regard to the safety and efficacy of the medicine.
Physicians need to be well-informed about the complex issues of bioequivalence and biosimilarity to feel confident about prescribing a new drug. Biosimilar products are not identical to their reference but similar, introducing a degree of uncertainty that may make the physician reluctant to switch. Questions may be raised as to what the differences between the biosimilar and reference product are, and what potential consequences may arise from these differences. This uncertainty can be reduced by proving the safety profile of both products.
Biosimilars: Licensed and in Registration; Three Classes of Therapeutic Proteins
As of May 2017, >30 biosimilars were licensed in Europe;30 they are segregated into three biologic classes. The first class of therapeutic proteins are substitution products, including hormone-like growth factors or insulin, which are relatively quickly absorbed (their effects are apparent within hours or days). The second class are proteins with a specific pharmacological effect, for example tumour necrosis factor-alpha inhibitors, whose effect is only visible after some time, with results not guaranteed in all patients. It is even harder to detect the clinical effects of the class three proteins, which include targeted therapies rituximab and trastuzumab. These products provide a statistical chance of benefit sometime in the future, for example extending the length of survival.31 The difficulty in observing clinical effect may have an effect on the market share over time, if prescribers do not have full confidence in the principles of biosimilarity (Figure 3).
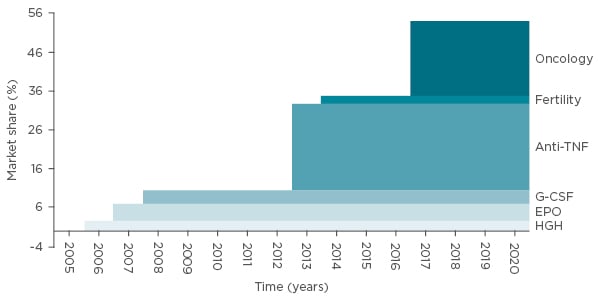
Figure 3: Size of therapy classes exposed to biosimilar competition (in sales) versus total European biologics market: Market share based on MAT 09 2015 sales values.32
EPO: erythropoietin; G-CSF: granulocyte colony stimulating factor; HGH: human growth hormone; TNF: tumour necrosis factor.
To improve uptake of Class 3 biosimilars, deep trust has to be developed in the principles of similarity, by analysing patient populations treated with biosimilars for similarity rather than clinical efficacy.
As of May 2017, 11 biosimilars were in EMA registration: two for adalimumab, two for bevacizumab, one for insulin glargine, two for pegfilgrastim, and four for trastuzumab.33 Having multiple biosimilars in the pipeline emphasises the need for physicians to be well-informed about current and emerging therapies in order to make the best therapy choice for their patients.
Manufacturing Challenges: Are Biologics Actually Biosimilars?
Biologics have complex manufacturing processes, with the key steps known only to the company manufacturing the original reference product, making them difficult to copy. Firstly, the cell line is created by cloning the gene for the therapeutic biologic molecule into the DNA vector and transfecting an appropriate host cell to express the protein. Cell numbers are then expanded through several cell culture processes, and the resulting product purified by purification and formulation protocols.34 Variable factors that have the potential to introduce changes to the resulting biologic product are present at every step of this process. The changing and optimising of manufacturing processes has always been an issue in the life cycle of biologics. Changes are categorised as low risk (e.g. changing filter supplier), moderate risk (e.g. moving to a new production facility), or high risk (e.g. using a new cell line).35 All changes mean that the new product has to be re-evaluated and scrutinised by the EMA to ensure the safety and efficacy of the product is maintained. The audience was polled on their confidence in the safety and efficacy of European or USA-approved biosimilars at the time of their initial launch. The results were split: approximately half of the delegates were moderately to fully confident about their use, while the remaining half would rather wait for more clinical data before recommending newly launched biosimilars.
The Role of New Molecule Innovation in the Sustainability of Treatment for Haematologic Malignancies
Professor Wojciech Jurczak
Prof Jurczak began by highlighting the scope of clinical trials conducted at Jagiellonian University, Krakow, Poland. Of the >50 ongoing lymphoma clinical trials, 2 of these are investigating biosimilars that are important for both patient and public finance outcomes. Healthcare professionals in Europe are accustomed to using biosimilars rather than their original products; for example, original epoetin and original G-CSF are now routinely replaced by biosimilars in everyday clinical practice.30
Rituximab: The Beginning of Monoclonal Antibody Therapeutics
Rituximab was the first monoclonal antibody to be approved for therapeutic use. Roche developed and registered the original intravenous rituximab molecule, followed by subcutaneous rituximab. More recently, rituximab biosimilars CT-P10 (Celltrion) and GP2013 (Sandoz) have been developed.
Rituximab is standard of care in first-line B-cell NHL, used as a first-line induction therapy in aggressive lymphomas, mantle cell lymphoma (MCL), iNHL, and CLL, and used as a first-line maintenance therapy in MCL, iNHL, and CLL.21 However, with new monoclonal antibodies and small molecules, the role of rituximab in relapsing/refractory NHL may be brought into question.
In the treatment of B-cell NHL, rituximab was described as ‘a great equaliser’ of chemotherapy regimens, illustrated by the similar overall survival (OS) outcomes in the CALGB/Alliance Phase III trial of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) versus dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) in untreated DLBCL.36 Likewise, Prof Jurczak noted that despite the availability of better monoclonal antibodies, such as obinutuzumab, OS outcomes were similar in the Phase III GOYA trial of R-CHOP versus obinutuzumab plus CHOP (G-CHOP) in untreated DLBCL,37 which suggests that chemotherapy may also be a great equaliser of monoclonal antibodies. Future therapies include the small molecules lenalidomide and ibrutinib, which are undergoing Phase III clinical trials in activated B-cell-like DLBCL. These compounds are used in addition to rituximab, rather than as a replacement.38,39 Rituximab also has a well-established role in treating relapsed/refractory DLBCL.40
Recommendations for the treatment of MCL will be published by the European Society for Medical Oncology (ESMO), based on Phase III maintenance studies.41-45 For young (<65 years), elderly (>65 years), and compromised patients, rituximab maintenance is included in first-line therapy, with targeted approaches (e.g. ibrutinib, lenalidomide, temsirolimus) reserved for first or multiple relapse.46
The Role of Rituximab in Indolent Lymphomas
Patients with iNHL may receive four to seven immune-chemotherapy lines of treatment in their lifetime. iNHLs are currently incurable, but the rate of therapy development progress suggests that patients can be treated now with the hope of access to better therapies in the future. Life expectancy for iNHL patients is expected to increase, possibly to the point where iNHL becomes a disease that patients live with rather than die from. This is proposed as a reason for holding back from initial use of high-tech chemotherapy and the best monoclonal antibodies, to maintain options in the future for relapsing/refractory disease. This is in contrast with CLL, where better monoclonal antibodies are used in first line with less intensive chemotherapy (namely, chlorambucil) in a subset of patients with comorbidities, with improved survival versus rituximab-chlorambucil.47
Maintenance rituximab as standard of care in iNHL was questioned at the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting. This is because it does not prolong OS and may induce adverse events. In addition, questions were raised about its cost effectiveness.48 However, maintenance therapy has an important role when less intensive chemotherapy is effective, so it can be used in iNHL patients who respond well to chemotherapy and who relapse late. This leaves an unmet medical need in iNHL patients who are refractory to first- line therapy or who relapse within 5 years.49 One of the key findings from ASCO 2017 was that the Phase III MAGNIFY clinical trial showed encouraging results from use of lenalidomide with rituximab in relapsed/refractory iNHL,50 a non-chemotherapy option in which rituximab still has a role.
Before and after Prof Jurczak’s presentation, the audience was asked “Have new anti-CD20 medicines displaced rituximab as the standard of care for B-cell malignancies?”. In the poll after his presentation, more of the audience agreed that rituximab is still the most effective and important agent in B-cell malignancies.
In conclusion, biosimilars have the potential to revolutionise cancer therapy by providing more affordable treatment for haematologic malignancies. If physicians, pharmacists, and payers are well informed about their use and convinced that the efficacy, safety, and quality of the biosimilars are comparable to their original reference products, biosimilar use will become widespread across Europe. The first anti-CD20 monoclonal antibody rituximab is still relevant in the treatment of B-cell lymphomas; two rituximab biosimilars are now licensed in Europe and have the potential to address gaps in care.
Click here to view the full symposium.







