Presenter: Pau Montesinos1,2
1. Hospital Universitari y Politècnic de La Fe, València, Spain
2. CIBERONC, Institute of Health Carlos III, Madrid, Spain
Disclosure: Dr Montesinos has received research support (to institution) from Celgene, Janssen, and Pfizer; consultation and participation in advisory boards for Celgene, Daiichi Sankyo, Novartis, Pfizer, AbbVie, Shire; is/has been a speakers bureau member for Otsuka Pharmaceutical, Celgene, and Daiichi Sankyo; and has received travel expenses from Amgen.
Acknowledgements: Medical writing assistance was provided by Dr George Xinarianos, OncoMed Communications & Consultancy Ltd, London, UK.
Support: The publication of this article was funded by Agios Pharmaceuticals, Inc.
Citation: EMJ Rheumatol. 2020;8[1]:47-49.
Summary:
Acute myeloid leukaemia (AML) commonly affects the elderly and is associated with a high risk of relapse and poor overall survival.1 AML patients who are ineligible for standard therapy based on advanced age and/or comorbidities have poor prognosis and limited treatment options;2,3 therefore, there is an unmet clinical need for certain subgroups of AML patients.
Isocitrate dehydrogenase 1 (IDH1) is a metabolic enzyme which catalyses the oxidative decarboxylation of isocitrate to α-ketoglutarate, while the mutant IDH1 (mIDH1) enzyme catalyses the reduction of α-ketoglutarate to the oncometabolite D-2-hydroxyglutarate.4,5 Accumulation of D-2-hydroxyglutarate results in myeloid differentiation arrest and epigenetic dysregulation, promoting leukaemogenesis.6-8 Somatic mutations in the IDH1 gene occur in 6–14% of patients with AML.9
In an ongoing Phase Ib study,10 23 newly diagnosed patients with mIDH1 AML were treated with ivosidenib 500 mg once daily, in combination with azacitidine 75 mg/m2 for 7 days (in a 28-day schedule). The spectrum of adverse events has been consistent with monotherapy experiences with ivosidenib or azacitidine. The investigators of the study reported four cases of IDH differentiation syndrome: three were
deemed to be serious adverse events, but all four cases resolved.
The overall response rate was 78.3% (18 patients) and 60.9% (14 patients) achieved a complete remission (CR). Median time to response was 1.8 months (range: 0.7–3.8 months) and median time to CR was 3.7 months (range: 0.8–15.7 months). Overall survival probability (12-month rate) was 82%. In patients with CR, mIDH1 clearance (<0.02–0.04%) in bone marrow mononuclear cells was observed in 71% (10 out of 14) of patients. These data provided the rationale for the design of the AGILE Phase III clinical trial.11
The authors report protocol amendments of key inclusion criteria and study design of the ongoing AGILE study of the ivosidenib plus azacitidine combination regimen in adults with mIDH1 newly diagnosed AML. AGILE is a global, double-blind, randomised, placebo-controlled, Phase III trial with a total of 166 participating study centres in North America, South America, Asia, and Europe. The study schema is shown in Figure 1.12 Enrolled patients were randomised 1:1 to receive either a combination of ivosidenib 500 mg once daily plus azacitidine 75 mg/m2 for 7 days in 28-day cycles, or a matched placebo plus azacitidine. Randomisation was stratified by region and by de novo versus secondary AML. Key eligibility criteria included patients with previously untreated mIDH1 AML, who were not candidates to receive induction chemotherapy (IC) treatment, and who had not received prior treatment with a hypomethylating agent or mIDH1 inhibitor. The recent amendment further specified criteria for IC-ineligibility as ≥75 years of age or reduced Eastern Cooperative Oncology Group performance status ([ECOG PS]: 2), or significant organ dysfunction (i.e., severe cardiac or pulmonary disorder, or impaired renal or liver function). The primary endpoint has been changed to event-free survival, defined as the time from randomisation until treatment failure, relapse from remission, or death from any cause (whichever occurs first). Treatment failure was defined as failure to achieve CR by Week 24. Key secondary efficacy endpoints were amended to overall survival, CR rate, CR plus CR with partial haematologic recovery rate, and overall response rate.
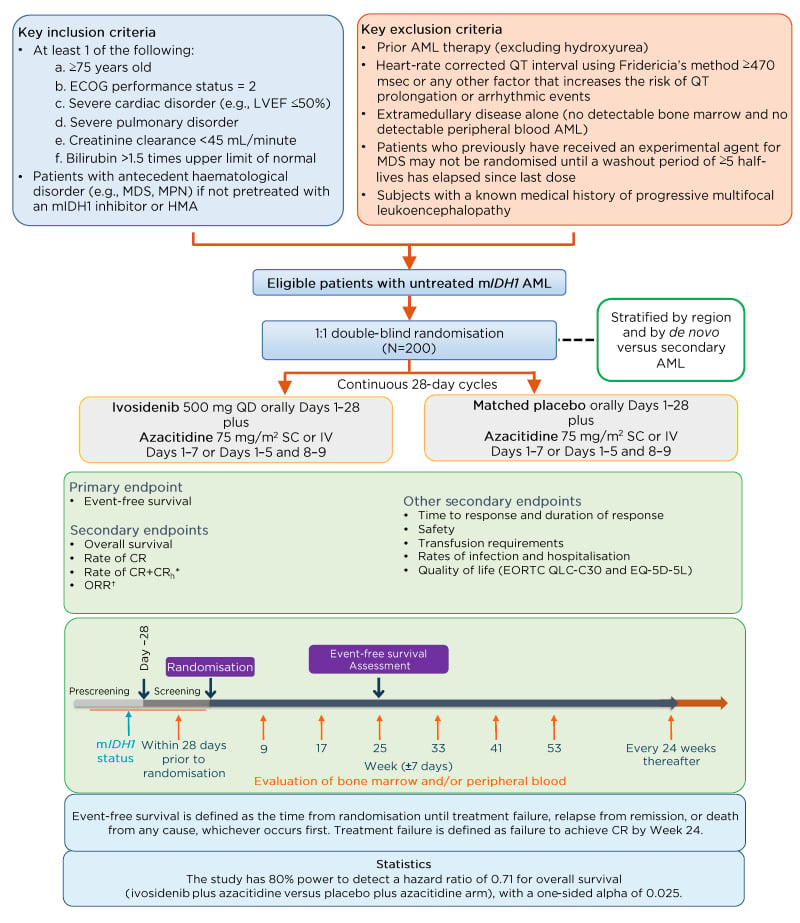
Figure 1: AGILE study schema.
*CRh is defined as CR with partial recovery of peripheral blood counts (<5% bone marrow blasts, platelets
>50,000 /μL, and ANC >500 /μL) and will be derived by the sponsor.
†Includes CR, CRi/CRp, partial response, and morphological leukaemia-free state.
AML: acute myeloid leukaemia; ANC: absolute neutrophil count; CR: complete remission; CRh: complete remission with partial haematologic recovery; CRi: complete remission with incomplete haematologic recovery; CRp: complete remission with incomplete platelet recovery; ECOG: Eastern Cooperative Oncology Group; EORTC QLQ-C30: European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire; EQ-5D-5L: EuroQol-5 dimension 5-level health-related quality of life questionnaire; LVEF: left ventricular ejection fraction; HMA: hypomethylating agent; IDH1: isocitrate dehydrogenase 1; IV: intravenous; MDS: myelodysplastic syndrome; mIDH1: mutant IDH1; MPN: myeloproliferative neoplasms; SC: subcutaneous; WHO: World Health Organization; QD: once daily.
Adapted from Montesinos et al.12
The favourable safety profile and encouraging clinical activity observed in the Phase Ib ivosidenib plus azacitidine combination study for the treatment of IC-ineligible mIDH1 AML warrant a timely and accurate assessment of the clinical benefit in this difficult-to-treat population with the Phase III AGILE clinical trial. AGILE is currently open for enrolment globally.