Meeting Summary
A recent symposium at the European Hematology Association (EHA) congress, chaired by Prof Eva Kimby, explored the changing paradigms in the treatment of non-Hodgkin’s lymphoma (NHL) and the potential impact of new approaches to diagnosis and treatment. Prof Kimby opened the symposium by discussing the recent therapeutic advances in the treatment of follicular lymphoma (FL). Prof Georg Lenz then spoke about the clinical implications of diffuse large B cell lymphoma (DLBCL) diagnosis and the manner in which disease subtyping can foster effective use of targeted therapies. Prof Catherine Thieblemont presented on post-induction treatment in DLBCL, and the importance of effective treatment options to limit the number of patients who fail first-line therapy. Prof Pier Luigi Zinzani then concluded the symposium by presenting data on the new immuno-oncology treatments being evaluated in patients with relapsed or refractory NHL.
Therapeutic Advances in Follicular Lymphoma
Professor Eva Kimby
The therapeutic management of FL is complicated by the disease heterogeneity, as evidenced by the differences in histological grading, prognostic factors (such as the FL International Prognostic Index [IPI], FLIPI2, and m7-FLIPI),1-3 and the blood and bone microenvironment, which can all vary widely between patients. Response to a specific treatment is also variable, as is the pattern of relapse. The cumulative effects of therapy such as cardiotoxicity, and the risk of transformation have to be considered when choosing therapy. IPIs are useful for predicting disease outcome at group level, but cannot identify a patient at low-risk of disease progression, in whom the benefits of immediate induction therapy are unclear. Indeed, data from the British National Lymphoma Investigation (BNLI) indicate that a ‘watchful waiting’ approach in low-risk patients is associated with a median time-to-initiation of chemotherapy of 2.6 years, and that 19% of patients still do not require chemotherapy after 10 years.4 More recent data also support the watchful waiting approach, with similar overall survival (OS) in patients receiving rituximab induction with maintenance, and those who undergo watchful waiting.5
The importance of rituximab maintenance in patients who show an initial response to first-line rituximab plus chemotherapy is well established.6 Data from the PRIMA trial7 indicated that only 18% of patients will have disease progression during an initial 2-year rituximab maintenance therapy period and that 75% of patients receiving rituximab-based maintenance therapy will remain progression-free at 3 years, compared with 58% of those undergoing observation (stratified hazard ratio: 0.55; 95% confidence interval: 0.44–0.68; p<0.0001). Although these benefits of rituximab are well established in patients with FL in the first-line setting, there remains substantial debate regarding the most effective accompanying chemotherapy regimen. A comparison of rituximab in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), or FM (fludarabine and mitoxantrone) indicates that rituximab-CHOP (R-CHOP) and rituximab-FM (R-FM) may yield an improved time-to-treatment failure compared with rituximab-CVP, but also that R-FM is associated with greater toxicity.8 Whether bendamustine plus rituximab offers an alternative to R-CHOP is unclear, although some data suggest that rituximab-bendamustine is associated with quality-of-life benefits and an improved toxicity profile.9,10 It is also unclear if chemotherapy is required in all patients, with data from Phase II and III trials suggesting that single-agent rituximab may also represent an alternative approach, albeit with a relatively short progression-free survival (PFS) of only 20–30 months.11,12 This is in contrast to the 3.5–7.4 years median PFS reported for patients on rituximab maintenance for 1–5 years.12 The probability of long-term survival following single-agent rituximab therapy is 87–89%, as observed in the SAKK35/03, PRIMA, and Nordic trials, respectively.7,11
There is also evidence supporting the combination use of rituximab with the immunomodulatory agent, lenalidomide,13 in patients with untreated indolent lymphoma. In an open-label Phase II study, patients with FL receiving rituximab plus lenalidomide (R2) achieved a response rate of 98%, a complete response (CR) rate of 87%, and >80% remained progression-free at 5 years.13 These data supported the subsequent initiation of the Phase II randomised R2-trial SAKK 35/10, in which rituximab was administered alone or in combination with lenalidomide to patients with untreated FL (n=77, each).14 At Week 23, overall response rates (ORR) were 82% in the combination arm, compared with 61% in the monotherapy arm (p=0.002), and with CR rates of 36% and 25%, respectively. An ongoing international study15 comparing first-line R2 versus rituximab-chemotherapy in >1,000 patients will provide further insight into the benefits of the R2 combination also in maintenance therapy.
New therapeutic agents are undergoing evaluation in patients with relapsing or rituximab-refractory FL (Figure 1). The GADOLIN study in patients with rituximab-refractory FL assessed the combination of bendamustine with obinutuzumab followed by obinutuzumab maintenance therapy versus bendamustine alone without any maintenance.16 Patients receiving the combination had improved PFS compared with those receiving bendamustine alone (median PFS not reached versus 14.9 months, p=0.0001) and there was evidence of benefits associated with obinutuzumab maintenance therapy compared with no maintenance therapy. Other new agents currently being studied in patients with relapsing or refractory disease are the PlK3-delta inhibitor, idelalisib, which has shown very high response rates in patients with refractory FL.17 Later trials of combinations of idelalisib with rituximab or bendamustine plus rituximab were halted due to toxicities.18 Ibrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor that has shown robust clinical activity when combined with rituximab in treatment-naïve patients.19 The BCL2 inhibitor, venetoclax, could be of particular interest in FL patients because of their over-expression of the BCL2 antiapoptotic protein.20 Checkpoint inhibitors such as the humanised anti-monoclonal antibodies, pidilizumab and nivolumab (PD1 inhibitors), and durvalumab (a PD-L1 inhibitor), represent another exciting class of immunotherapy undergoing evaluation in patients with FL.
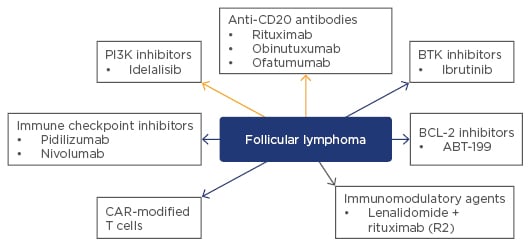
Figure 1: Novel and upcoming non-cytotoxic treatments* for patients with follicular lymphoma.
*Rituximab is approved as a single agent and in combination with chemotherapy for initial and maintenance treatment of follicular CD20-positive non-Hodgkin’s lymphoma. Idelalisib is approved for relapsed/refractory follicular lymphoma. All other agents listed in Figure 1 are investigational.
BTK: Bruton’s tyrosine kinase; CAR: chimeric antigen receptor; BCL-2: B cell lymphoma-2.
Further development of these interesting new agents would be facilitated by an early surrogate marker of PFS. In the current clinical trial environment, most first-line treatments will have a long remission period and patients can frequently have subsequent repeated relapses. The Follicular Lymphoma Analysis of Surrogate Hypotheses (FLASH) group has assessed individual patient data from 11 randomised clinical trials and identified the presence of a CR at 30 months (CR30) as a surrogacy candidate for PFS.21 CR30 captures the effects of both induction and maintenance treatments, and is supported by clinical data indicating that durable CR is associated with prolonged PFS.22 Incorporation of markers such as CR30 into future clinical trial design may permit a reduction in overall clinical trial duration.
Novel Insights into Diffuse Large B Cell Lymphoma Diagnosis and Clinical Implications
Professor Georg Lenz
Existing diagnostic procedures for DLBCL are well established. A lymph node or extranodal biopsy is followed by morphological and immunohistochemistry (IHC) characterisation. IHC can also be employed for disease subtyping, fluorescent in situ hybridisation analysis for identification of translocations, and computed tomography or positron emission tomography (PET), and potentially bone marrow biopsy, for clinical staging. Within this diagnostic process, defining the DLBCL disease subtype is important because of the wide spectrum of heterogeneous disease. It is possible to distinguish a number of morphologic variants (centroblastic, immunoblastic, anaplastic large B cell, plasmablastic, etc.) each with specific morphology and pathology, and there is also wide heterogeneity with regard to disease manifestation (nodal DLBCL versus mediastinal, central nervous system [CNS], or testicular lymphomas) and treatment response.
Gene expression profiling (GEP) can help in resolving DLBCL heterogeneity by reliably and reproducibly distinguishing the activated B cell like (ABC) subtype (35% of DLBCLs); the germinal centre B cell like (GCB) subtype (40%); and the primary mediastinal B cell lymphoma subtype.23 Importantly however, 15–20% of all DLBCLs cannot be classified according to GEP and are considered to represent a mixture of different lymphomas. Unfortunately, the clinical use of GEP is limited by the requirement for fresh frozen biopsies and therefore IHC algorithms have also been evaluated for clinical applicability. The use of IHC for disease subtyping is also subject to practical limitations, most notably the inability to identify the 15–20% of patients who are unclassifiable through GEP. IHC disease subtyping therefore identifies patients only as GCB or non-GCB.24 Another approach to disease subtyping is the NanoString GEP assay, which is compatible with fresh frozen tissue and may therefore have utility within the clinical setting. Overall, disease subtyping is important because the DLBCL molecular subtypes rely of different oncogenic pathways: disease subtyping can therefore facilitate the rational use of targeted therapies.
The nuclear factor (NF)-κB pathway is one such oncogenic pathway that is utilised differently between ABCs and GCBs. NF-κB is a transcription factor family that is normally inactivated by inhibitory proteins but upon stimulation causes the release of NF-κB into the nucleus, resulting in cell proliferation and inhibition of apoptosis. In tumour cells, the NF-κB pathway can be constitutively activated and pre-clinical data suggest that the ABC subtype may be particularly reliant on this this oncogenic pathway.25 Ibrutinib and lenalidomide are inhibitors of the NF-κB pathway and both have shown activity in patients with ABC DLBCL. Ibrutinib is a high specific inhibitor of BTK, which plays an important role in activating the NF-κB pathway. It has shown better response rates in patients with ABC compared with GCB (37% versus 5%, p=0.0106), which also translated into a trend towards improved OS in the ABC group (median OS: 10.32 months in ABC versus 3.35 months in GCB, p=0.056).26 Ibrutinib responses appear to be dependent on the presence of specific NF-κB pathway mutations within CD79B, MYD88, the CARD11 coiled coil domain, and TNF-α-IP3 (Figure 2). Lenalidomide also shows preferential activity in ABC compared with GCB DLBCL. A retrospective analysis has shown higher response rates in non-GCB patients compared with GCB patients with relapsed/refractory DLBCL receiving lenalidomide, resulting in significantly improved PFS in the non-GCB group.27 Further evidence comes from a retrospective analysis of a Phase II study of R-CHOP alone or with lenalidomide in patients with DLBCL. Among patients receiving R-CHOP alone, OS was significantly better among GCB patients compared with non-GCB patients; however, in the combination therapy arm there was no difference in OS between GCB and non-GCB groups, suggesting a lenalidomide-driven improvement in OS in the non-GCB population.28 Further data on these agents will come from the ongoing Phase III studies ROBUST29 and PHOENIX,30 which will assess the combination use of ibrutinib or lenalidomide with R-CHOP in patients with non-GCB DLBCL.
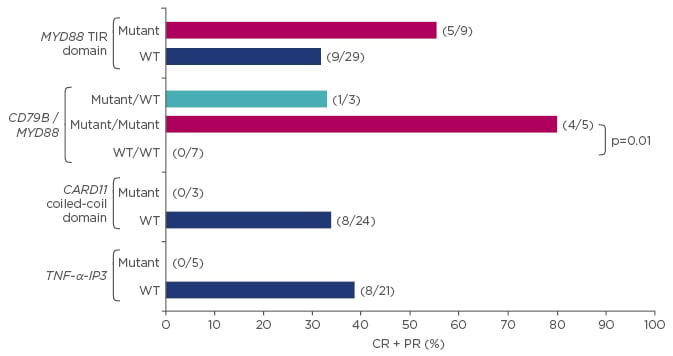
Figure 2: Ibrutinib shows improved survival benefit in patients with activated B cell like versus germinal centre B cell like diffuse large B cell lymphoma.26
*All other: 12/35, 10/32, 14/30, respectively. p-value versus other: 1.00, 0.057, 0.031, respectively.
CR: complete response; PR: partial response; TNF-α-IP3: tumour necrosis factor alpha-induced protein 3; WT: wild-type.
Although patients with GCB typically respond well to R-CHOP, there is also a need for additional targeted treatment options for these patients. In vitro evidence suggests that the PI3 kinase (PI3K) pathway may represent a rational drug target for GCB DLBCL.31 Expression levels of the phosphatase and tensin homolog (PTEN) protein, which normally blocks the PI3K pathway and therefore acts as a tumour suppressor, are lower in GCB compared with non-GCB patients; the addition of PTEN induces toxicity in PTEN-deficient GCB DLBCL cell lines and inhibition of PI3K results in toxicity in PTEN-deficient models.
For the foreseeable future, R-CHOP is likely to remain the standard treatment choice in DLBCL; however, improved understanding of the oncogenic pathways in each molecular subtype may precipitate a movement towards increased use of targeted therapies. ABCs and GCBs are clearly different tumours characterised by different GEPs and different genetic abnormalities. The ability to distinguish ABC and GCB in the clinical setting will be the first step towards specific treatment approaches for each molecular subtype.
Post-Induction Treatment in Diffuse Large B Cell Lymphoma: Current Data and Perspectives
Professor Catherine Thieblemont
In patients with DLBCL, first-line R-CHOP therapy results in a clinical cure in approximately 60% of treated patients while the remaining 40% typically relapse within 2 years. Survival in patients who relapse is poor: those with late relapse (~half) have a median survival of approximately 5 years and those with early relapse (i.e. within 1 year, ~half) typically of less than 6 months.32 Based on these data, it is clear that DLBCL is a ‘one-shot’ cancer with poor prognosis following relapse. Effective management approaches for preventing relapse are therefore required, raising two important questions: ‘what are the specific characteristics of relapsed patients?’ and ‘how can these patients be effectively treated?’ Patients with refractory disease can be identified based on the clinical features of the disease (the site of involvement, e.g. CNS disease and the IPI); their immunophenotypic characteristics (such as Ki67); biological characteristics (such as MYC or the double hit); gene expression signatures (GCB/ABC, microenvironment, stromal signatures); and their treatment response based on PET analysis.33
For patients who will relapse, the R-CHOP induction regimen is clearly insufficient and additional treatment is required. Various Phase III studies have examined replacement of CHOP with intensified chemotherapy regimens such as etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (CHO[E]P or EPOCH) or doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (ACVBP), or the use of high-dose therapy plus autologous stem cell transplantation (ASCT) as consolidation.34 Studies examining intensified chemotherapy regimens have generally not yielded improved survival rates compared with CHOP.31 Alternatively, replacement of rituximab by another antibody such as ofatumumab or obinutuzumab may represent another approach to mitigating relapse. A third approach has therefore been pursued which involves an additional drug treatment to the R-CHOP regimen, either during the induction treatment phase or as a maintenance therapy. The rationale for the maintenance therapy approach is to prevent post-remission relapse in patients with a first complete remission after induction therapy. This approach has been successfully adopted with new agents in the treatment of other cancers such as multiple myeloma, where lenalidomide maintenance therapy following ASCT has been shown to significantly prolong OS.35
The assessment of minimal residual disease (MRD) based on circulating tumour DNA may be one approach to evaluating the benefit of maintenance therapy. Only one study has reported that after three cycles of first-line therapy, the presence of circulating tumour DNA is associated with reduced PFS and OS. Strategies to decrease MRD using maintenance therapy may therefore delay or prevent relapse.36 While studies suggest no benefit associated with rituximab or everolimus maintenance therapy in DLBCL,37,38 data in support of lenalidomide maintenance therapy are more promising. Notably, a Phase II study in chemosensitive patients with relapsed DLBCL receiving lenalidomide maintenance therapy after a complete or partial response to rituximab-based salvage therapy indicated a favourable survival advantage.39 Of the 41 patients enrolled in this study, 30 had DLBCL and 11 had transformed DLBCL. The 1-year PFS was 74% and 1-year OS was 84%. Encouragingly, 6 of the 16 patients with a partial response after salvage therapy achieved a CR during lenalidomide maintenance. In this study, lenalidomide had a predictable toxicity profile; 22% of patients developed Grade 3/4 neutropenia. Lenalidomide is also currently under evaluation as first-line maintenance therapy in the randomised Phase III REMARC study in patients with DLBCL and response following R-CHOP induction.40
In conclusion, maintenance therapy approaches may help to reduce or prevent relapse and improve treatment outcomes for patients with DLBCL. Evaluation of MRD may help to adapt this treatment approach specifically for DLBCL patients and studies are ongoing in this regard. Data from the REMARC study of maintenance lenalidomide after R-CHOP in elderly patients are also eagerly awaited.
New Frontiers in Immuno-oncology
Professor Pier Luigi Zinzani
Novel immunotherapeutic agents represent exciting new treatment modalities for relapsed/refractory NHL. At the forefront of research are a number of different strategies that utilise the immune system to promote tumour destruction. The pleiotropic pathway modifier, CC-122, exhibits both tumouricidal and anti-angiogenic effects in vitro (Figure 3), and shows excellent immunomodulatory activity compared with lenalidomide.41 Whereas lenalidomide appears to be more effective in the ABC subtype, preliminary in vitro data suggest that CC-122 is active in both ABC and GCB subtypes, implying therapeutic potential in a wider cross-section of patients.42 This agent is currently undergoing evaluation in patients with NHL, both as a single agent and also in combination with rituximab, ibrutinib, or obinutuzumab. Studies examining CC-122 in triple therapy regimens include combinations with rituximab plus a mammalian target of rapamycin inhibitor, or rituximab plus a BTK inhibitor.
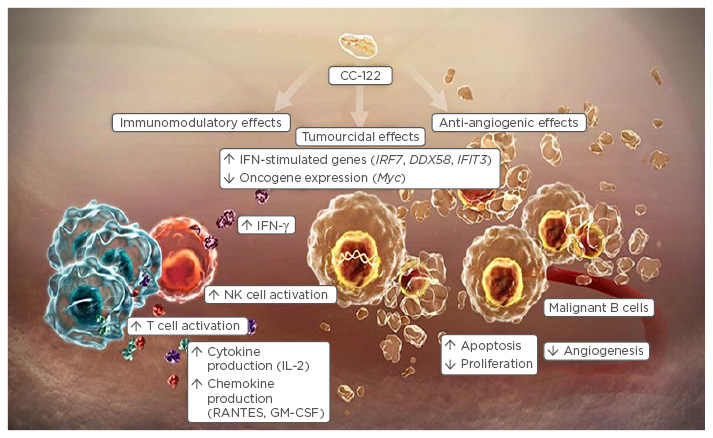
Figure 3: Mechanism of action of the pleiotropic pathway modifier, CC-122.
IFN-γ: interferon gamma; NK: natural killer; IL: interleukin; GM-CSF: granulocyte-macrophage colony-stimulating factor; RANTES: regulated on activation, normal T cell expressed and secreted.
Taken with permission from http://www.researchoncology.com/
A number of new monoclonal antibody-drug conjugates (ADC) are also undergoing evaluation in patients with relapsed or refractory NHL. The ROMULUS study compared two ADCs, pinatuzumab vedotin (a CD22 ADC) and polatuzumab vedotin (a CD79b ADC), each in combination with rituximab, in 41 patients with relapsed or refractory FL.43 Most patients in this study were heavily pre-treated and all had received prior rituximab: 43% of those in the pinatuzumab and 25% of those in the polatuzumab arms became rituximab refractory within 6 months. Neurotoxicity was reported in both treatment arms, with 50–60% of patients reporting peripheral neuropathy or peripheral sensory neuropathy. CRs were 40% and 10% in the polatuzumab and pinatuzumab arms, with ORR of 70% and 62%, respectively. On the basis of these results, ongoing Phase II studies are evaluating combinations of polatuzumab plus rituximab plus CHOP and pinatuzumab plus rituximab plus bendamustine.
Denintuzumab mafodotin is another ADC under evaluation in relapsed/refractory NHL. It has a similar mechanism of action to polatuzumab and pinatuzumab (an anti-CD19 monoclonal antibody), but with a different vinca alkaloid cytotoxic agent (monomethyl auristatin F instead of monomethyl auristatin E) which confers a different toxicity profile.44 A Phase I study including 54 patients with primarily relapsed/refractory DLBCL evaluated 3-weekly (0.5–6 mg/kg every 3 weeks) and 6-weekly (3 mg/kg every 6 weeks) dose regimens of single-agent denintuzumab mafodotin. Ocular toxicity, including blurred vision, dry eye, fatigue, keratopathy, and photophobia were the main safety observations, and, similar to polatuzumab and pinatuzumab, there was little evidence of significant haematological toxicity. The ORR was 37% with the 3-weekly regimen and 44% with the 6-weekly regimen, with CRs achieved in 20% and 44% of patients, respectively. These data are considered preliminary and the ocular toxicity profile suggests further study of this agent is required.
Checkpoint inhibitors represent another exciting therapeutic approach to the treatment of aggressive NHL. Nivolumab has shown activity in patients with previously treated NHL and pembrolizumab in those with relapsed/refractory Hodgkin’s lymphoma. A Phase II study of the PD-1 inhibitor pidilizumab reported ORR of 51% in patients with relapsed/refractory DLBCL; and a Phase I study of single-agent nivolumab response rates of 28%, 36%, and 40% in patients with relapsed/refractory indolent non-follicular B cell lymphoma (n=29), DLBCL (n=11), and FL (n=10), respectively.44,45 Checkpoint inhibitors also appear to have an acceptable safety profile with no concerning haematological or stomatological toxicity, and no clear association between pneumonitis and prior therapies.46 Finally, durvalumab, a selective high-affinity human immunoglobulin G monoclonal antibody that blocks PD-L1,47 is also beginning clinical evaluation in a Phase Ib/II2 study, both as a monotherapy and as a combination therapy in patients with lymphoma and chronic lymphocytic leukaemia.48
Chimeric antigen receptor (CAR)-modified T cells are known to be effective in treating relapsed and refractory acute and chronic lymphocytic leukaemia, and it has been hypothesised that CAR-modified T cells directed against CD19 may result in anti-tumour responses in patients with advanced CD19+ B cell NHL.49 Initial data from a Phase II study which enrolled patients with relapsed/refractory CD19+ NHL suggest promising anti-tumour activity. Following a single intravenous infusion, ORR was 47% in patients with DLBCL and 73% in those with FL, with CRs achieved in 20% and 36% of patients, respectively.49 The safety and anti-tumour activity of another CD19+ targeted CAR-T cell, JCAR017, is also being evaluated in a Phase I study in patients with relapsed/ refractory NHL.50
In conclusion, immunotherapy is advancing at a spectacular rate; its role in NHL continues to evolve and early phase clinical trial results are promising. New compounds with immunomodulatory activity, monoclonal antibodies, checkpoint inhibitors, and CAR-T cell therapy have yielded interesting preliminary data in DLBCL and FL. These new immunological approaches have the potential to improve treatment outcomes, and further clinical evaluation will help define their role in the treatment of patients with relapsed/refractory NHL.







