Abstract
The management of acute lymphoblastic leukaemia (ALL) remains challenging. The changing landscape of newer agents and combinations of chemotherapy are improving outcomes, and various conditioning regimens and possible donor sources for allogeneic transplant provide management options; allograft remains the most potent anti-leukaemia therapy available. With improvements in treatments and monitoring of disease response, allogeneic transplantation is becoming more refined as an important option for selective patients with difficult disease. Although the paediatric ALL protocols used for adolescents and young adults are now extended towards the middle-aged patients, and newer therapeutic agents may be incorporated, there is evolving data comparing short and long-term outcomes and deliverability of treatment. Reliance on registry transplant data is inadequate in guiding optimal therapy for the individual, who may have a variety of specific needs. With the limited clinical trials in this field, it is important to continue reviewing progress and outcomes with alternative stem cell sources, such as mismatched unrelated donors, haploidentical donors, and cord blood transplants, which may cure many patients, though carry risks of treatment-related mortality and morbidity. Conditioning regimens of reduced toxicity have enabled the older and higher risk patients to proceed to allograft, but it remains hazardous. It is important to understand the features of the malignant cells, response to therapies, individual patient factors, donor stem cells available, and patient’s wishes, to help craft the current management. Allogeneic transplantation remains a very important option for ALL, and patient selection and path to transplant are continuing to evolve and be guided by ongoing clinical and laboratory data, including minimal residual disease assessment.
BACKGROUND
Acute lymphoblastic leukaemia (ALL) is an aggressive malignancy with various subtypes, generally associated with a very poor outlook without the use of therapy. Whilst seen as the most common childhood malignancy, the incidence of ALL is low and recorded as 300 cases per year in Australia, 750 cases per year in the UK, and 6,000 cases per year in the USA; approximately 60% of these cases are in children.1-3 B-lineage is predominant in cases of ALL, with a ratio of approximately three to one compared to T-lineage.4 Since the 1950s, progress has continued, with paediatric and adult ALL research groups working on successive trials with ongoing major improvements in outcomes and improved results.5-12 In adult ALL, there are differences in the disease biology that exist, including the frequency of Philadelphia positive disease.4,13 The time to recovery after chemotherapy is longer in the older patient, and the toxicity to organs such as the lungs, liver, and gut may be more troublesome and, thus, reduces the ability to deliver the scheduled therapy.14 Doses may need to be reduced or omitted; some important chemotherapeutic agents such as asparaginase may cause major toxicity, such as pancreatitis, hepatitis, hyperlipidaemia, and thrombosis, particularly in patients over the age of 50 years.15
The favourable outcomes for adolescents and young adults have been demonstrated by improved results with the paediatric protocols in retrospective studies, including an event-free survival at 39 months of 67% (versus 39%).16 This has resulted in extending the more aggressive paediatric regimens to adults, resulting in good response rates and outcomes (Table 1), such as a 3-year overall survival of 69%.17 However, with associated improvements, there have been concerns about the toxicity of the paediatric regimens.18 Effective primary therapy reduces the need for allografts, although it is important to try to predict those patients who are likely to subsequently relapse because such cases have the best chance for long-term survival by using allograft in first remission. Later attempts at allograft may be unsuccessful. An established risk factor for ALL is age >60 years, where allogeneic transplantation is not generally offered as a routine procedure. As well as presenting high blast counts and Philadelphia chromosome, cytogenetic changes of chromosomal translocations t(4;11)19 and t(1;19)20 and delays in achieving remission are associated with poorer outcomes.21 The Philadelphia chromosome is the most common cytogenetic abnormality in adult ALL, comprising 20–30% of adult cases but only 3–5% of paediatric cases.22
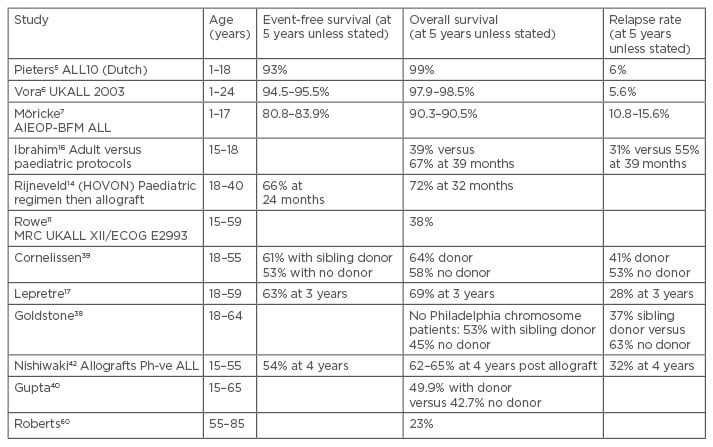
Table 1: ALL studies highlighting poorer outcomes with advancing age, and some benefits of allografts.
ALL: acute lymphoblastic leukaemia; Ph-ve: Philadelphia-negative.
Allogeneic transplantation has been used for ALL for >40 years. It is established as the most effective anti-leukaemia therapy and has been used for patients with high-risk disease such as primary refractory, relapsed, or Philadelphia positive ALL (who had a short survival prior to the availability of tyrosine kinase inhibitors [TKI], with improvements subsequently).23,24 Various stratifications have been formulated to guide such decisions, and persisting minimal residual disease (MRD) detection after treatment appears to be particularly helpful as a powerful predictor of transplants that are destined to fail.25,26
CONSIDERATIONS OF ALLOGENEIC TRANSPLANTATION IN ACUTE LYMPHOBLASTIC LEUKAEMIA
Patient Factors in Allogeneic Transplantation
There are many issues to consider when possible allograft is considered in the ALL patient. These include the age of the patient, as highlighted by outcomes in Table 2; performance status; past medical history; active comorbidities; and current medications.
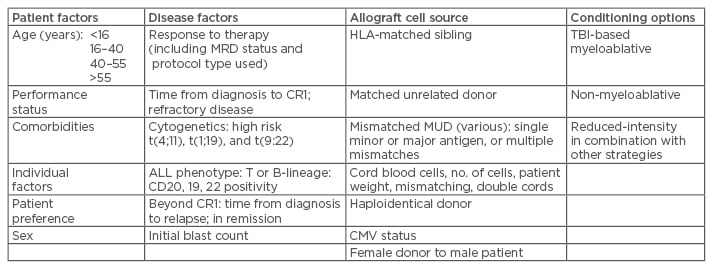
Table 2: Considerations for allogeneic transplant for ALL.
Young patients attaining prompt MRD: defer allograft.
Adults with adverse disease factors, including high-risk cytogenetics, suboptimal response to therapy, and MRD positivity: advise allograft for optimal disease control, considering stem cell source, conditioning (TBI-based <50 years), and patient factors.
Patients relapsing beyond CR1: aim to regain disease control and patient fitness, progressing towards allograft using best available source of stem cells and TBI-based conditioning where feasible in patients <50 years old.
ALL: acute lymphoblastic leukaemia; MRD: minimal residual disease; TBI: total body irradiation; CR1: first complete remission; HLA: human leukocyte antigen; MUD: matched unrelated donor; CMV: cytomegalovirus.
In the paediatric population, with the excellent results with initial chemotherapy and monitoring of response, the role of allograft has changed, even in high-risk diseases, such as Philadelphia positive ALL.27
Of particular importance is the individual patient history of recent infections, including fungal, HIV, cytomegalovirus, hepatitis viruses, cardiac disease and reduced ventricular function, respiratory disease, renal impairment, and diabetes. The inability of the patient to receive planned treatment (such as with a paediatric protocol) may influence management; the long-term outcomes for reduced-dose chemotherapy will be compromised in the individual, suggesting the best chance for long-term survival may be with transplant.
Patient factors are considered, including intensity of conditioning and optimal medications to control underlying issues. Assessments may be characterised formally, such as the European Group for Blood and Marrow Transplantation (EBMT) risk score, which includes patient age, disease status, and time from diagnosis over performance status (as well as donor factors).28
Other areas for consideration are the psychological and social factors of the individual. A history of major depression or psychosis, illicit drug use, non-compliance, or failing to attend appointments or take prescribed medications may be a prodromal warning of high risks of morbidity and mortality from allograft. Concerns about compliance and adequate social support have been identified as significant issues, though allografts have been successfully performed on such patients.29 Also of major importance is the patient’s informed consent, following counselling on various short-term, long-term, and individual factors, outlining the uncertain path of each graft recipient. Initial conditioning and depth of remission prior to allograft appear important, with the combination of a paediatric chemotherapy regimen prior to allograft providing encouraging results.14
Disease Factors and Allografts
Ongoing efforts continue to identify factors of the malignant cells and the environment, in attempts to forecast the likely success or failure of intensive chemotherapy. Previously established unfavourable features, including cytogenetics and presenting blast counts for disease subtypes, have been used to identify higher risk diseases that may not be cured by chemotherapy; such cases have been related to allografts as seen in Table 2. More recently, the response to initial chemotherapy and detection of MRD have been strongly predictive of those likely to progress to frank relapse. This allows the identification, by using flow cytometry25 or next-generation sequencing,26 of those who may have the highest chance of long-term survival with allograft. Monitoring of disease with quantitative polymerase chain reaction, such as for Philadelphia positive disease with the BCR-ABL fusion gene, helps greatly in directing response to therapy and guiding management, including the use of TKI. Although results combining TKI and paediatric protocol chemotherapy have been promising in children,27 in adults the relapse-free survival was inferior compared to allografts (though overall survival was not statistically different at 5 years).30 Apart from the detection of residual disease by flow cytometry, understanding surface markers of the malignant cells also provides the opportunity to use specific agents to target disease, such as rituximab,31 inotuzumab ozogamicin,32 and blinotumomab.33 The latter has been used as a bridge towards allograft for some relapsed or refractory patients (Table 3).
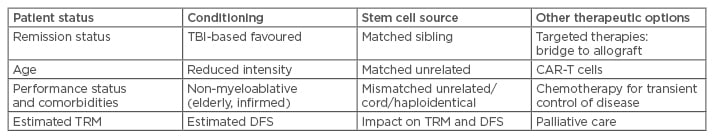
Table 3: Considerations with allografts for ALL in advanced disease.
ALL: acute lymphoblastic leukaemia; TBI: total body irradiation; TRM: treatment-related mortality; DFS: disease-free survival; CAR: chimeric antigen receptor.
A recent paper compared allograft in ALL patients in first remission with those having deferred allograft. Three-year event-free survival was only 28% in the deferred group, but many were salvaged with delayed allografts in second remission (MRD-negative), concluding similar overall survival in both groups.34 This highlights the use of MRD testing, as it has been demonstrated as the most important factor to stratify ALL relapse risk, including excellent outcomes for those obtaining early rapid responses to chemotherapy.35 In a German study,35 limited outcomes of 32.6% 5-year disease-free survival for those with significant MRD were detected before consolidation therapy, suggesting allograft may be favoured in this group. Also, in adult T cell-ALL patients receiving allogeneic stem cell transfer in first complete remission (CR1), MRD positivity pre-transplant did not adversely affect outcome, unlike those treated with chemotherapy alone.36 This highlights the value of allograft in selected patients.
Allografts in First Remission of Acute Lymphoblastic Leukaemia Versus Allografts in More Advanced Disease or Primary Refractory Disease
It is established from registry data that patient outcomes are superior when the allografts are performed in CR1 with limited outcomes for those with more advanced disease.37 This approach has been supported for standard risk disease by a previous large study,38 prior to the adoption of the paediatric protocols in the adult patients, though the optimal strategy for CR1 has been debated, with toxicity of treatment concerns. An earlier study to evaluate ALL patients in CR1 demonstrated superiority for those with matched siblings who, when proceeding to allograft, had a 60% 5-year disease-free survival rate, compared to 42% for those without matched siblings (who received autografts).39 The reduced relapse rate was 24% at 5 years for the allograft group, though the non-relapse mortality was estimated at 16%. Also, on a worldwide meta-analysis, sibling donor myeloablative transplant was found to improve survival only for patients under the age of 35 years, due to higher treatment-related mortality in the older group.40 Conversely, some patients may be receiving curative chemotherapy but are taken to allograft and do not survive, or have significant unwanted morbidities. Patients obtaining rapid clearance of disease (measured by MRD) have excellent results without allograft;35 conversely, adults with positive MRD35 or high-risk cytogenetics, such as Philadelphia positive disease,30 may be best managed by proceeding to allograft in CR1. For primary refractory disease, allograft can provide reasonable results in some subsets,41 with some long-term survivors in this group with otherwise poor outcomes.
The difficulties with delayed allograft beyond first remission are that many patients may fail to obtain disease control or will succumb to complications prior to the proposed allograft. Even for those who do achieve satisfactory disease control and physical status to proceed to allograft, the results have been limited.37 Hopefully, newer agents may provide better depth of disease control to reduce relapse rates and optimise the situation pre or post-transplant.
Selection of Allogeneic Stem Cells, Possible Donors, and Stem Cell Sources
Finding a suitable donor is paramount prior to any discussion about proceeding to allogeneic transplant. Prompt searching is undertaken shortly after diagnosis in prospective patients, although only 20–30% will have a human leukocyte antigen (HLA)-matched sibling. There are alternative sources of haematopoietic stem cell transplantation in children and adults with ALL who need an allogeneic transplant but lack a suitable sibling donor. Registry searches achieve variable success with factors, such as racial background, impacting on the chance of identifying a suitable volunteer unrelated donor. However, long-term outcomes are similar to matched sibling donors if a search for cord blood cells is performed concurrently, with multiple cords being considered when stem cell numbers are limited, such as in adults.42 Currently, haploidentical donors identify a further source of donor cells for allograft with the advantages of prompt availability and low costs. Suboptimal donors with mismatches are associated with inferior outcomes, including higher rates of graft versus host disease.43 Pleasingly, results similar to sibling allografts have been recently published with cord transplants as well as haploidentical stem cells, although the matched sibling has been generally viewed as the optimal source of donor cells.44,45 For adults, the longstanding hierarchy of donor cells has been that HLA-matched siblings are favoured, then well-matched unrelated donors, then either cord blood cells or mismatched unrelated donors or haploidentical donors. Individual factors, including the need to progress promptly and availability, as well as transplant centre preference, may direct choices, but there are no published direct comparisons of these cell sources. For the paediatric population, results with related and other cord transplantations have been well established as a suitable alternative, with less graft versus host disease.46
Decisions on Conditioning Regimens
Total body irradiation combined with chemotherapy has been established as a standard conditioning regimen for ALL with evidence of reduced relapse compared to chemotherapy alone,47 although treatment-related mortality has been troublesome and other options have been explored.48 Generally, patients over the age of 55 years have been deemed unsuitable for this treatment due to excessive toxicity. Reduced intensity and non-myeloablative conditioning enable some older patients and those with significant comorbidities to progress to allograft, but may be associated with higher relapse rates, although this is not shown in all studies.49 A single-centre study showed promising results for reduced intensity conditioning in combination with TKI for Philadelphia positive ALL.50 In acute myeloid leukaemia, the disease-free survival was found to be superior with myeloablative transplants compared to reduced treatment intensity, and this also appears to be the case in ALL.47,51
Ongoing efforts to limit treatment-related mortality and, also, to improve longer-term quality of life include reducing graft versus host disease by including the use of anti-interleukin 6 therapy with tociluzumab,52 the use of mesenchymal stromal cells,53 as well as reducing infections and sinusoidal obstructive syndrome with newer agents. There are ongoing attempts to target disease to reduce relapse rates, including optimising the depth of remission in second complete remission with preceding therapies.41
Other Treatment Options as an Alternative to Allograft
Unfortunately, for adults with relapsed or refractory ALL, the outlook is poor.54 Attaining MRD-negative remission prior to allograft can provide long-term disease control.25,26 As the outcome with chemotherapy in paediatric patients has continued to improve in association with effective large collaborative trials, the role of allogeneic transplantation has reduced in children.8 It appears that with the adaptation of the paediatric-based regimens in the younger adult patients with associated improvement in outcomes, allogeneic transplantation in first remission may decline. Adjunctive therapies, such as rituximab in CD20 positive B cell ALL patients, may also assist older patients with comorbidities, suboptimal donor sources of available stem cells, and other factors. The lack of head to head studies and limited collaborative trials makes it difficult to provide clear recommendations.
Late relapses in the chemotherapy-treated patients do occur, which can limit long-term success without allograft, and there are currently various options for relapsed or refractory patients. For patients with CD19 positive B-lineage ALL, the option of blinatumomab may provide disease control with some patients having prolonged survival, of which some may proceed to allogeneic transplant.55 Currently, it is not seen as a single agent for long-term disease control. Similarly, there is increasing data on chimeric antigen receptor T cells,56,57 meaning they may also be seen as an alternative salvage therapy, with high response rates. Various types of chimeric antigen receptor T cells and schedules have been developed that may improve outcomes and reduce major problems, including cytokine release syndrome and neurological issues.58 High response rates without acute graft versus host disease have been seen in a small series for B cell-ALL relapse post allograft.59 Another agent, inotuzumab ozogamicin, for advanced CD22 positive ALL has a continuing Phase III study.32 As these newer therapies emerge, longer-term survival may rival disease-free survival of allograft, and without the issues of graft versus host disease and other long-term toxicities from transplant (and conditioning). However, the elderly ALL patients remain a challenge.60 Novel immune-based and molecular therapies may be of major benefit in some subtypes of ALL, particularly in combination with existing regimens.
SUMMARY
Allograft remains the most potent anti-leukaemia therapy available, and is recommended in CR1 with ongoing MRD positivity and selected high-risk disease, such as adverse cytogenetics, where a matched sibling donor is available or a suitable alternative donor, including haploidentical or cord blood. Also, for refractory disease and in second complete remission, allograft should be pursued as part of a curative strategy in other advanced cases, including in older patients. Although toxicities and complications limit outcomes, reduced intensity conditioning regimens minimise treatment-related mortality, at the risk of higher relapse rates. Pleasing results with cord blood and haploidentical cells for allogeneic transplant provide the management option of transplantation for most patients, although outcomes may be inferior to matched sibling donors. Using immunological and molecular agents in combination with chemotherapy is improving outcomes. There is a gradation of risk as the patient becomes older, both for the risks of relapse and the risks of treatment. Comorbidities, the presence of MRD, the source of donor cells, the risks of transplant, including conditioning, and other options must be considered for selecting best management of the individual.
We strive to extend results from paediatrics, where most ALL patients obtain a long-term cure with current strategies. Adopting these protocols also appears to reduce the survival benefits of allogeneic transplant for the younger adults, to enable a more selective approach. The effectiveness of the initial chemotherapy regimen and disease monitoring influences the decision of whether or not to proceed to allograft in CR1. For adult patients who have Philadelphia positive ALL, allograft is currently standard therapy, but in the elderly the combination of TKI and chemotherapy may provide reasonable outcomes together with molecular monitoring. Thus, the suitability for allogeneic transplantation requires consideration of a variety of patient and disease factors, and future scoring systems may assist. For patients with persisting or recurrent MRD detection, frank relapse, or established high-risk disease, proceeding towards allogeneic transplantation remains standard. Recent studies have confirmed satisfactory outcomes with the alternative stem cell sources of cord blood and haploidentical donors, enabling this curative strategy to be available to almost all patients. Ongoing studies of outcomes will continue to shape future management.







