Abstract
Anaemia is the most widespread of the haematological disorders, affecting about one-third of the global population. Despite decades of public health interventions, anaemia in pregnancy remains a major health problem worldwide, with an estimated 41.8% of pregnant women being diagnosed with anaemia at some point in their gestation. At least half of the cases of anaemia in pregnant women are assumed to be due to iron deficiency, with folate or vitamin B12 deficiency, chronic inflammatory disorders, parasitic infections like malaria, and certain inherited disorders accounting for the remaining cases. A considerable variation has been observed in the incidence and aetiology of iron deficiency anaemia among developed and developing nations, warranting differences in the screening protocols and management strategies used by clinicians in these countries. This article highlights the differences in the management of iron deficiency anaemia among low and high-income countries, with a detailed review of the policies followed in India.
DEFINING ANAEMIA IN PREGNANCY
The definition of iron deficiency anaemia (IDA) in pregnancy is imprecise as a result of pregnancy-induced changes in plasma volume and haematocrit, differences in haemoglobin (Hb) concentration through the trimesters, differences in diagnostic tests, and ethnic variation. According to the World Health Organization (WHO), a pregnant woman is considered to be anaemic if her Hb concentration is <11 g/dL,1 whereas Centers for Disease Control and Prevention (CDC) guidelines define anaemia as Hb <11 g/dL in the first trimester and <10 g/dL in the second or third trimester.2 Based on the finding of typically lower Hb and haematocrit levels among black adults, the Institute of Medicine (IOM) recommends lowering the Hb cut-off level by 0.8 g/dL in this population.3 The British Committee for Standards in Haematology (BCSH)4 defines anaemia as a Hb value <2 standard deviations below the mean value for a healthy matched population, which amounts to Hb levels <11.0 g/dL in the first trimester, <10.5 g/dL in the second to third trimesters, and <10.0 g/dL in the postpartum period.
Around three-quarters of anaemia-related maternal mortality cases in south Asia occur in India, and hence the Indian Council of Medical Research (ICMR) further classifies anaemia in pregnancy according to grades of severity to aid in the triage of women requiring early intervention:5 mild anaemia with Hb of 10.0–10.9 g/dL,moderate anaemia with Hb of 9.9–7.0 g/dL, severe anaemia with Hb of 6.9–4.0 g/dL, and very severe anaemia with Hb of <4.0 g/dL.
PATHOPHYSIOLOGY OF IRON DEFICIENCY ANAEMIA IN PREGNANCY
Physiological or dilutional anaemia of pregnancy is observed in healthy pregnant women as a result of a relatively greater expansion of plasma volume by 30–40% in comparison to a 20–25% raise in Hb mass and erythrocyte volume. This results in a modest decrease in Hb levels, creating a low viscosity state, which promotes oxygen transport to the placenta and fetus. Pregnancy significantly increases the demand for iron to balance the physiological requirements of increased haematocrit, developing the fetoplacental unit, and for losses during delivery and lactation. The Institute of Medicine (IOM) estimated that the total iron loss associated with pregnancy and lactation is approximately 1,000 mg and has recommended a daily dietary allowance for iron in pregnancy of 27 mg instead of the 8 mg required for the nonpregnant adult population.6 If this recommended daily allowance of iron in pregnancy is not met, it results in women with depleted iron stores developing IDA.
Recent research into iron metabolism in humans has paved the way for the discovery of a novel peptide hormone, hepcidin, that acts as a homeostatic regulator of systemic iron concentration by controlling iron efflux into the plasma. Hepcidin levels in pregnant women are, in general, lower than in nonpregnant healthy women, and decrease as pregnancy advances, with the lowest levels of hepcidin observed in the third trimester.7 With decreasing expression of hepcidin, there is an increased absorption of dietary iron and increased mobilisation of iron from body stores modulated by ferroportin. Inflammatory states, including pre-eclampsia, malaria infection, and obesity, have been associated with higher hepcidin during pregnancy compared to healthy controls, suggesting that maternal and fetal iron bioavailability could be compromised in these conditions.
INCIDENCE OF IRON DEFICIENCY ANAEMIA IN DEVELOPED VERSUS DEVELOPING COUNTRIES
IDA remains the most common nutritional deficiency globally, with about 32 million pregnant women categorised as anaemic and about 0.75 million pregnant women categorised as severely anaemic.8
An analysis of National Health and Nutrition Examination Survey (NHANES) epidemiological data from the USA from 1999–2006 demonstrated an overall prevalence of IDA of nearly 18.0%. Iron deficiency was shown to increase from 6.9% to 14.3% to 28.4% across the three trimesters during pregnancy.9 A multicentre cross-sectional study in the UK estimated a 24.4% prevalence of maternal anaemia at some stage of the antenatal period.10
A systematic analysis of population- representative data of Hb concentration and the prevalence of total and severe anaemia for 1995–2011 reported the prevalence of anaemia in pregnancy as 14.0% in high-income regions and 23.0% in central and eastern Europe.11 In contrast to these developed countries, about 53.0% of pregnant women in south Asia were diagnosed with anaemia, of whom 3.8% were found to be severely anaemic. Iron-amenable anaemia represented >70.0% of these cases.11 Insufficient quantity of iron-rich foods, poor environmental sanitation, unsafe drinking water, iron loss due to parasite load (e.g., malaria or intestinal worms), and adolescent anaemia, along with teenage pregnancies and repeated pregnancies in low resource countries, are the predominant causes for the disproportionately increased prevalence of IDA in pregnancy in these nations.
India is one of the countries with the highest prevalence of anaemia in the world. According to the Indian National Family Health Survey, the prevalence of IDA in pregnancy ranges from 23.6–61.4%.12 The incidence of IDA in India was estimated at 60.0% in the urban population and 69.0% in the rural population, and IDA resulted in approximately 326,000 maternal deaths with an associated disability-adjusted life years of 12,497,000.13 Diverse cultures, religions, food habits, lifestyles, and traditions pose a challenge to the implementation of various government health programmes in India.
The situation is no different in other Asian countries, like Pakistan, where in 2008 the prevalence of anaemia among pregnant women was estimated at 90.5%; of these women, 75.0% had mild anaemia and 14.8% had moderate anaemia.14 In the year 2000, a community-based sample of 336 pregnant women in the plains of Nepal showed that 72.6% of pregnant women were anaemic and 88.0% of cases of anaemia were associated with iron deficiency.15
MATERNAL AND FETAL EFFECTS OF IRON-DEPENDENT ANAEMIA IN PREGNANCY
The existing literature shows that failure to meet the increased iron requirements in pregnancy may result in adverse maternal and fetal consequences. This is also supported by a comparative quantification of health risks by the WHO, which estimated that about 591,000 perinatal deaths and 115,000 maternal deaths worldwide can be attributed to IDA, either directly or indirectly.16 Furthermore, it was shown that correcting IDA of any severity reduces the risk of maternal death by about 20% for each 1 g/dL increase in Hb. In light of these findings, newer health policies need to focus on mild-to-moderate anaemia in pregnancy, in addition to severe anaemia, for a greater public health impact.
Iron deficiency in pregnancy causes maternal morbidity with increased risk of abortion, increased susceptibility to infection due to defective macrophage phagocytosis and lymphocyte replication, physical weakness, pre-eclampsia, preterm labour, heart failure, increased risk of postpartum haemorrhage due to impaired myometrial contractility resulting from hypoxia-induced enzymatic and cellular dysfunction, puerperal sepsis, and postnatal depression. According to a study by Lone et al.,17 anaemia in pregnancy increases the risk of preterm birth 4.0-fold, low birth weight babies 2.2-fold, and low Apgar score in newborn babies 1.8-fold in comparison to nonanaemic women. Maternal iron depletion also reduces fetal iron stores and increases the risk of neonatal anaemia and perinatal morbidity. Correction of iron deficiency has proved to have beneficial effects on both the mother and the fetus. A meta-analysis by Haider et al.18 demonstrated a dose–response relationship between increasing doses of iron supplements and reduction in the incidence of low birth weight babies.
SCREENING IN DEVELOPED COUNTRIES VERSUS DEVELOPING COUNTRIES
Strategies for anaemia screening may include a routine screening of all expectant mothers or targeted screening based on established risk factors, diagnostic tests, or risk assessment instruments. Though the need for antenatal screening is well established, countries differ from one another in their screening policies and criteria.
The U.S. Department of Veterans Affairs/Department of Defence (VA/DoD) and CDC2 recommend that all pregnant women should be screened for anaemia at some point during pregnancy, irrespective of their risk stratification. The VA/DoD recommends screening during the first antenatal visit and is against routine repeat screening in asymptomatic pregnant women.19 In comparison, the American College of Obstetricians and Gynaecologists (ACOG) recommends routine screening of all pregnant women for anaemia and implementing iron therapy if IDA is confirmed.20
Australian guidelines recommend the screening of all pregnant women for anaemia at the time of booking and at 28 weeks.21,22 If the pregnant woman is at risk of thalassaemia or haemoglobinopathy, she will require additional investigations, such as Hb variant analysis and iron studies.
The IOM recommends that screening for anaemia should be reserved for high-risk pregnant women only, who need to be followed up during each trimester and at 4–6 weeks postpartum.3 The U.S. Preventive Services Task Force concluded that the current evidence is inadequate to assess the balance of benefits and harms of screening for IDA in pregnant women to prevent adverse maternal and fetal outcomes. It also stated that although there is insufficient evidence to prove the superiority of any screening tool, measurement of serum Hb or haematocrit levels may often be considered as the first step used in primary care practice. The Canadian Task Force on Preventive Health Care, on the other hand, does not have a current recommendation for this topic.23,24
With >80% of antenatal women diagnosed with IDA, routine screening of all pregnant women is standard practice in India. Indian National Rural Health Mission (NRHM) guidelines recommend a compulsory Hb estimation for all pregnant women by the cyanmethaemoglobin method or by photocalorimeter at 14–16 weeks, followed by at 20–24 weeks, 26–30 weeks, and 30–34 weeks of pregnancy (a minimum of four Hb estimations). The interval between the Hb estimations should be a minimum of 4 weeks. The trigger point for referral to a more specialised institution would be a Hb level of ≤7 g/dL at 14 weeks, 20–24 weeks, and 26–30 weeks, or a Hb level of ≤9 g/dL at 30–34 weeks.25
WORK-UP
Clinical Symptoms and Signs
Symptoms and signs of IDA in pregnancy are usually nonspecific and mimic normal pregnancy changes, unless the anaemia is severe. Fatigue is the most common symptom, followed by varying degrees of pallor, lassitude, headache, palpitations, dizziness, dyspnoea, lack of concentration, and irritability. Pica develops in rare cases.
Full Blood Count, Blood Film, and Red Cell Indices
A complete blood count is usually the primary step in the diagnosis of IDA. It is simple, rapid to perform, inexpensive, and helpful in the early prediction of IDA. A full blood count provides a complete blood picture, showing low Hb, mean cell Hb (MCH), mean cell volume (MCV), and mean cell Hb concentration (MCHC); a peripheral smear with presence of microcytic hypochromic red cells and characteristic ‘pencil cells’ or anisopoikilocytosis characterises IDA.
Red Cell Distribution Width
Increased red cell distribution width implies variance in the red blood cell (RBC) volume distribution, similar to a peripheral blood smear anisocytosis, and helps in differentiating IDA from thalassaemia and other haemoglobinopathies. The sensitivity and specificity, respectively, of red cell distribution width in the diagnosis of IDA in pregnancy were reported by Sultana et al.26 as 97.4% and 83.2% and by Tiwari et al.27 as 72.8% and 82.4%.
Serum Ferritin
Measurement of serum ferritin accurately reflects iron stores and is commonly the first laboratory test to become abnormal as iron stores decrease. Serum ferritin values are not affected by recent iron ingestion and are generally considered the best parameter to assess deficient iron stores in pregnancy. A concentration <15 μg/L indicates iron depletion in all stages of pregnancy. Assessment of serum ferritin in pregnancy takes precedence over other investigations in women with suspected thalassaemia or haemoglobinopathy, in women whose anaemia fails to respond to a 2-week trial of oral iron, in women with multifactorial anaemia, and before any parenteral iron replacement.
Serum Iron, Total Iron Binding Capacity, and Transferrin Saturation
Serum iron is an unreliable indicator of the iron available at the tissue level because of wide fluctuations in serum iron levels with recent ingestion of iron, infection, and diurnal rhythm. Total iron binding capacity is increased with iron deficiency and is an indirect measure of obtainability of iron-binding sites and transferrin levels.
Zinc Protoporphyrin
Zinc protoporphyrin levels increase when iron availability decreases as zinc is incorporated into the protoporphyrin ring instead of iron. Serum zinc protoporphyrin has the advantage of not being influenced by the plasma dilution and hence has greater sensitivity and specificity for iron depletion; however, this test is rarely performed because it is not readily available.
Bone Marrow Iron
A bone marrow sample stained for iron with Prussian blue has been considered the gold standard for assessment of marrow iron stores. However, the invasive nature of this test restricts its use to the most complicated cases, in which the underlying cause of anaemia cannot be identified by simpler means.
A Trial of Iron Therapy
A trial of iron therapy has simultaneous diagnostic and therapeutic applications in IDA. Microcytic hypochromic anaemia can be assumed to be due to iron deficiency until proven otherwise. A rise in Hb level, if demonstrable by 2 weeks, confirms iron deficiency and is both cost and time-effective. If the patient is at high risk of haemoglobinopathy and the status is unknown, they can be evaluated with ferritin and iron therapy can be started while screening is being performed. If there has been no improvement in Hb by 2 weeks, referral should be made to more specialised centres to consider other causes of anaemia.
WHO and CDC technical guidance is that, in the absence of infection, measuring serum ferritin or serum transferrin receptor in association with Hb provides the best assessment of iron status in the general population.28 However, in low-resource settings like India, the majority of these tests are either not easily affordable or not available. Therefore, the RBC indices hold great value for primary diagnosis, which can reduce unnecessary investigative costs. Of all the available indices, the Meltzer index (MCV:RBC ratio) has been shown to be the most reliable indicator with the highest sensitivity.29
According to Indian National Rural Health Mission guidelines,25 management of IDA in pregnancy includes:
- Hb estimation by cyanmethemoglobin method using a semiautoanalyser or photocalorimeter, which is mandatory in all institutions.
- Peripheral smear, MCV:RBC ratio, serum iron binding capacity, and Hb electrophoresis performed in medical colleges, District Headquarter hospitals, and other secondary care institutions with facilities for these tests.
- Urine assessment for albumin, sugar, and deposits, and a urine culture if pus cells are detected, to rule out refractory anaemia.
Supplementation and Prophylaxis
All antenatal women must be given advice for dietary modification to improve iron content and its absorption. Iron-rich food items include meat from cattle, fish, and poultry, and legumes and green leafy vegetables. The recommended dietary intake of iron in the second half of pregnancy is 30 mg. Absorption of iron increases 3-fold by the third trimester and the requirement increases from 1–2 mg to 6 mg per day.30 This increase cannot be taken care of by dietary modification alone and hence results in anaemia during pregnancy in many women. The WHO strongly recommends daily oral iron and folic acid supplementation as a part of antenatal care to reduce the risk of low birth weight babies, maternal anaemia, and iron deficiency. The International Nutritional Anemia Consultative Group (INACG), as well as the WHO, recommends that 60 mg elemental iron has to be given as prophylaxis to all antenatal women in countries where the prevalence of anaemia is >40%.1
In the UK and Australia, routine administration of iron supplementation to all pregnant women is not recommended. However, in India, where prevalence is as high as 58%, the Ministry of Health and Family Welfare (MOHFW) recommends iron supplementation in the form of 100 mg elemental iron for 100 days along with 500 μg of folic acid starting from 14–16 weeks. However, the problem with 100 mg elemental iron compared to 60 mg is increased incidence of gastrointestinal side effects like nausea, vomiting, and constipation. For nonanaemic patients with iron deficiency, the dose can be as low as 20–60 mg.
According to a recent Cochrane review,31 iron supplementation reduces the risk of anaemia in full-term mothers by 70% and the risk of iron deficiency by 57%. However, there was no significant effect on preterm birth and neonatal death. The fetus is relatively protected from the effects of iron deficiency by upregulation of placental iron transport proteins.31 To improve acceptability of adherence to supplementation, a behavioural change communication strategy should be implemented to communicate the benefits of oral iron supplementation. This is consolidated by the provision of an information leaflet in the patient’s language in the UK. In India, nutrition counselling is provided at antenatal check-ups during the monthly village health and nutrition day. Accredited social health workers are given incentives for providing iron folic acid supplements to patients at their doorstep.
The available ferrous salts include ferrous fumarate, ferrous sulphate, ferrous gluconate, and succinate. The amount of elemental iron varies in each salt and hence the number of tablets to be taken daily varies; for instance, if a woman takes ferrous gluconate she needs to take two tablets per day, as compared to ferrous sulphate or ferrous fumarate (one tablet per day), as shown in Table 1.
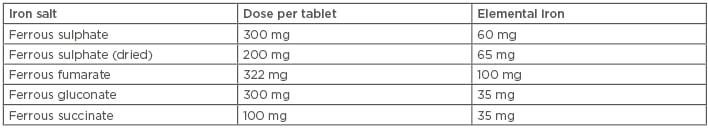
Table 1: Elemental iron content and dose per tablet of oral iron preparations.
Oral iron supplementation should be taken on an empty stomach along with vitamin C-containing food items and women should be informed not to have tea, coffee, or antacids when taking iron tablets.
Supplementation with oral iron is continued for 3 months, and a therapeutic dose of iron is started if the anaemia is not corrected. In populations with endemic hookworm (a prevalence of ≥20–30%), antihelminthic therapy should be given to any patient with severe anaemia because treatment is safer and cheaper than diagnosing a hookworm infection. After the first trimester, mebendazole or albendazole can be safety administered to pregnant women. In India, all pregnant women are given one tablet of 400 mg albendazole at 14–16 weeks. In malaria endemic areas, provision of iron folic acid supplementation should be implemented in addition to measures for prevention, diagnosis, and treatment of malaria.32
TREATMENT
National Institute for Health and Care Excellence (NICE), British Society of Haematology (BSH), and Australian Guidelines all recommend a trial of oral iron for 2 weeks in women diagnosed with anaemia during the antenatal period.4 This treatment can be started at the community level and an adequate rise in Hb is considered to be diagnostic of IDA.21,22,34 If the haemoglobinopathy state is unknown, iron therapy should be started and a haemoglobinopathy screen should be organised simultaneously. Duration of therapy should be the time until the patient’s Hb rises to normal and then a further 3 months or until 6 weeks postpartum in order to replenish stores. If Hb is <10 g/dL in the postpartum period, 100–200 mg elemental iron should be given for a further 3 months. Nonanaemic iron-deficient women should be given 65 mg elemental iron and Hb test should be repeated after 8 weeks.
Supplementation compliance has to be ensured in patients. For example, women should be asked about the colour of their stools, which should be black while on oral iron treatment, or they can be asked to return the empty supplement packaging. Gastrointestinal side effects can be as high as 40% with oral iron therapy, and include nausea and vomiting, constipation, and diarrhoea. Particularly if the mother is near term, the indications of referral to secondary care in the UK are:
- No rise in Hb during 2 weeks of treatment.
- Hb <7 g/dL.
- Symptoms of iron supplementation.
- >34 weeks into pregnancy.
Absorption of oral iron can be increased by taking a source of vitamin C along with the supplement on an empty stomach. Parenteral iron should be considered from the second treatment month onwards in patients with:
- Severe gastrointestinal side effects.
- Intolerance to oral iron malabsorption.
- IDA unresponsive to oral iron.
- Absolute noncompliance, particularly if mother is near term.
Among the various parenteral iron preparations, iron carboxymaltose is the most preferred drug, and there is no need to give a test dose because it is rarely associated with anaphylaxis. This drug can be given as total drug infusion and is available at the concentration of 50 mg/mL of elemental iron. The dose is calculated on the basis of pre-pregnancy or booking weight, aiming for a target Hb of 11 g/dL.
Infused over 15 minutes, 1,000 mg in 20 mL is diluted in 250 mL of 0.9% sodium chloride. The patient is observed for 30 minutes after administration and oral iron is avoided for the next 5 days. Hb testing is repeated after 2–3 weeks and a second dose can be planned if required. A single dose should not exceed 1,000 mg of iron per week. A general practitioner has to be notified if continued iron therapy is needed.
In India, oral iron therapy is initially trialled in all women with Hb ≤9 g/dL. In patients with Hb 7–9 g/dL, parental iron should be considered after 32–34 weeks for an early rise, ensuring 100% compliance. In patients with Hb <7 g/dL, parenteral iron should be considered. The government of India recommends the use of intravenous iron sucrose since it is available readily and cheaper than alternatives.26 It is available at a concentration of 20 mg/mL and is given as a slow intravenous injection of 100 mg or infusion of 200 mg. The U.S. Food and Drug Administration (FDA) approves a maximum dose of parenteral iron of 600 mg per week. Unlike ferric carboxymaltose, it cannot be given as a total dose infusion. Contraindications for parenteral iron are hypersensitivity to iron or any cause other than IDA.
Among intramuscular preparations, the only one available in the UK is low molecular weight iron dextran. In India, the most popular iron preparation is iron sorbitol citrate complex; however, these injections tend to be painful and cause permanent skin staining. Their use is therefore generally discouraged and no longer recommended in the UK. If one is to be given, the Z-track injection technique should be used.
Women who are still anaemic at the time of birth may require additional precautions, such as:
- Birth in a hospital setting.
- Group and save of blood.
- Active management of the third stage.
- A plan to deal with postpartum haemorrhage.
The need for blood transfusion can arise in the following situations:
- Severe anaemia in the last trimester for immediate improvement in Hb status.
- Severe anaemia with signs of cardiac failure or hypoxia.
- Haemoglobinopathy or bone marrow failure syndromes.
- Acute haemorrhage: if Hb <6 g/dL or if the patient becomes haemodynamically unstable due to ongoing haemorrhage.
The recent Royal College of Obstetricians and Gynaecologists (RCOG) (Figure 1) blood transfusion guideline33 recommends blood transfusion in labour or the immediate postpartum period if Hb is <7 g/dL. In Western countries, provision of cell salvage should be considered at the time of caesarean section.
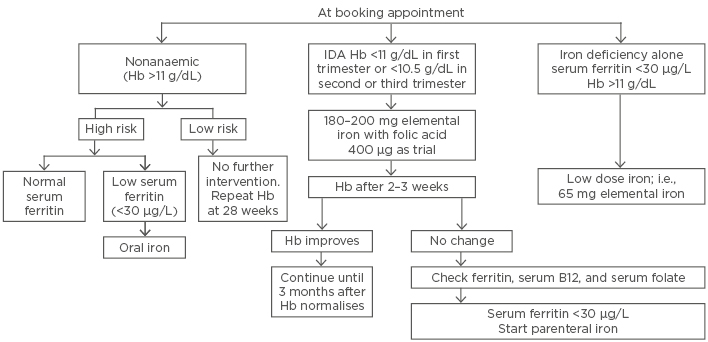
Figure 1: Management of iron deficiency anaemia in pregnancy in accordance to Royal College of Obstetricians and Gynaecologists (RCOG) Green-top Guidelines.
Hb: haemoglobin; IDA: iron deficiency anaemia.
Adapted from Blood Transfusion in Obstetrics: Green-top Guideline No. 4731
CONCLUSION
IDA during pregnancy continues to be a major health problem in the developing world. This warrants certain steps at the individual and community levels, such as education of pregnant women about anaemia, its causes, and health implications. Nutritional education, with a special emphasis on strategies based on locally available foodstuffs, administration of appropriate iron and folate supplements while ensuring maximum compliance, treatment of chronic disease like malaria deworming, and universal antenatal care to pregnant women will help in combatting this serious health hazard. Long-term governmental policies should be directed towards formulation of effective plans to eradicate anaemia in children and adolescent girls. Owing to the significant heterogeneity in screening, diagnosis, and treatment of IDA across nations, more research is needed to understand the clinical effects of routine screening, the ideal screening tools, and the most effective treatment of IDA during pregnancy.







