Abstract
Myelodysplastic syndromes are a group of disorders that affect the bone marrow, subsequently affecting the growth and relative abundance of blood-forming cells in the circulating volume. Myelodysplastic syndromes often do not cause early signs or symptoms, and can be found during routine blood tests. Granulocyte colony-stimulating factors (G-CSF) have been used in the treatment of myelodysplastic syndromes with neutropenia. Filgrastim, a G-CSF, helps increase the number of circulating neutrophils. Therefore, it has been proven to reduce patient vulnerability to infections in instances such as chemotherapy-induced neutropenia. This case report describes a 66-year-old male who presented for a pre-operative assessment before an elective left total hip arthroplasty. Routine bloodwork showed a low neutrophil count, and the surgery was cancelled due to concerns about the patient’s risk of infection. Further testing included a bone marrow aspirate and core biopsy that showed mild megaloblastic erythropoiesis and a relative increase in the proportion of myeloblasts and promyelocytes. The patient was given a working diagnosis of early myelodysplasia, and a trial of a low-dose G-CSF was started. The neutrophil count was monitored at 6–72 hours. After 72 hours of administration of filgrastim, the patient’s blood neutrophil levels had improved outside the range of neutropenia. After clearance for surgery, the patient had a successful hip arthroplasty with no post-operative infection reported. No neutropenia was noted post-surgery. This case highlights the potential of filgrastim to be used as prophylaxis before an elective surgery to improve moderate neutropenia related to primary myelodysplasia.
Key Points
1. Myelodysplastic syndromes cause blood dyscrasias, such as neutropenia, leaving patients vulnerable to infection during elective surgeries.2. Granulocyte colony-stimulating factors treat myelodysplastic syndromes by increasing the number of white blood cells in the bone marrow.
3. Filgrastim, a granulocyte colony-stimulating factor, can be used as prophylaxis before elective surgeries to improve moderate neutropenia related to primary myelodysplasia.
BACKGROUND
Myelodysplastic syndromes (MDS) are a group of blood disorders that occur due to ineffective haematopoiesis that results in abnormal blood-forming cells in the bone marrow. Neutropenia, or having a lower-than-normal level of neutrophils, has been associated with an increased risk of infection in myelodysplastic syndromes.1,2 Granulocyte colony-stimulating factors (G-CSF) help the bone marrow increase the number of white blood cells and are used to treat MDS for neutropenic complications.3-7 Filgrastim, a GCS-F, has been used in the treatment of neutropenia resulting from many causes. The side effects of this medication include alopecia, bone and joint pain, and gastrointestinal issues. As a prophylactic means for moderate neutropenia, filgrastim has not been thoroughly studied to determine its feasibility in improving neutropenia.
CASE PRESENTATION
A 66-year-old male presented to his family physician for a routine pre-operative evaluation for an elective left total hip arthroplasty. He was otherwise asymptomatic and did not report a history of recurrent infections. His past medical history was remarkable for hyperlipidaemia and prostate cancer with radical prostatectomy (Table 1).
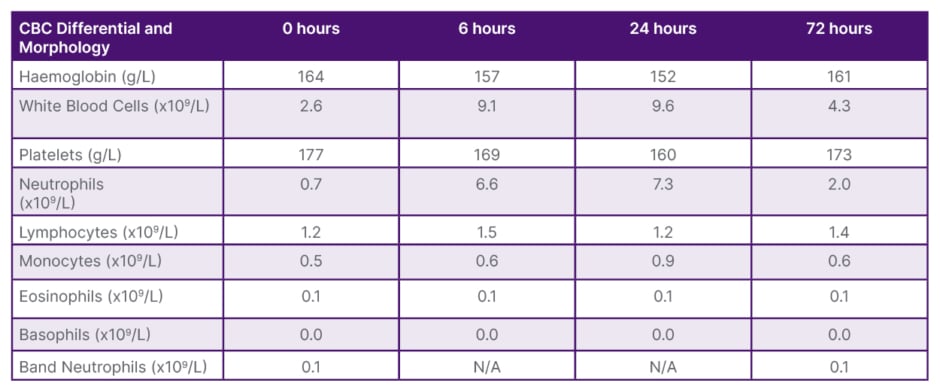
Table 1: Complete Blood Count Differential and Morphology values 6–72 hours after administration of filgrastim.
CBC: complete blood count; N/A: not applicable.
His surgery was cancelled due to the orthopaedic surgeon’s concern that the risk of infection potentially outweighed the benefit of surgery, and the patient was referred to Haematology. Haematology reviewed the patient and completed a left posterior iliac crest bone marrow aspirate and core biopsy. The biopsy showed normocellular marrow with trilineage haematopoiesis showing mild megaloblastic erythropoiesis and left shifted granulopoiesis. The associated molecular and cytogenetic studies on the bone marrow aspirate were unremarkable. The working diagnosis from Haematology was early myelodysplasia.
The patient was determined to get the elective hip arthroplasty due to a change in functioning, including requiring a walking aid, and inability to do activities that he enjoyed, such as golf. The haematologist discussed a trial of low-dose G-CSF 300 µg subcutaneously for infection prophylaxis for elective hip arthroplasty, including risks and benefits, and the need to trend the neutrophil count at 6–72 hours to assess its effectiveness. The patient consented to a trial of this medication for its off-label use.
DISCUSSION
A low-dose G-CSF has been shown to improve neutropenia associated with MDS. In one randomised controlled Phase III trial, one such G-CSF, filgrastim, was shown to be safe and effective in increasing blood neutrophil levels for patients with congenital, cyclic, and idiopathic neutropenia.3 Furthermore, in that trial, patients reported an improved quality of life due to a reduced frequency of fevers, inflammation, and infections. In addition, at a conventional dose of 3 mg/kg/d of a granulocyte-macrophage colony-stimulating factor, the incidence of severe infections was reduced, and moderate neutropenia due to MDS was improved.8
A novel approach was taken in this patient by trailing a low-dose G-CSF subcutaneously for neutropenia based on the working diagnosis of early myelodysplasia. In this case, for the patient, there was a perceived risk of infection due to the elective arthroplasty and therefore, the surgery was cancelled. The patient still sought to continue with the procedure, after consideration of the benefits and risks, to improve his functioning. Therefore, a trial of a low-dose G-CSF was perceived to improve the patient’s neutrophils levels. Unfortunately, the G-CSF was not covered by the Saskatchewan Exceptional Drug Formulary without a formal haeme-oncological diagnosis, and the medication had to be covered out of pocket.
The neutrophil values along with other values from the Complete Blood Count Differential and Morphology taken at 6, 24, and 72 hours are provided in Table 1. After 6 hours on filgrastim, the patient’s neutrophil levels improved significantly from 0.70×109/L to 6.6×109/L. This increase in absolute neutrophil count meant that the patient was outside the range of even mild neutropenia.9 At 24 hours, the blood neutrophil count had further increased to 7.3×109/L, resulting in an above normal neutrophil count. At the final bloodwork draw, taken at 72 hours after administration of filgrastim, blood neutrophils levels decreased to 2.0×109/L. After this trial of filgrastim, the patient was cleared to undergo the elective procedure, and the surgery proceeded successfully. Neutrophil values along with other selected Complete Blood Count Differential and Morphology values 1 week prior to his surgery and post-surgery are presented in Table 1. Twenty four hours post-surgery, the neutrophil values were outside the range of neutropenia, and the patient showed no signs of infection. This trend suggests a potential role for filgrastim to treat moderate neutropenia. Further studies are warranted to determine if a low-dose G-CSF should be considered in a prophylactic regimen to treat moderate neutropenia and reduce infection risk prior to surgery.
CONCLUSION
Neutropenia, because of myelodysplastic syndromes, presents a potential risk of infection following surgery. Filgrastim, a G-CSF, is a special protein that stimulates the bone marrow to produce neutrophils to fight infection. It has been used to reduce febrile neutropenia, infection rates, and hospitalisation in patients receiving myelosuppressive chemotherapy.8 This report details the case of a patient with moderate neutropenia prior to undergoing elective arthroplasty. Due to the neutropenia, the surgery was cancelled. With a working diagnosis of early myelodysplasia, a low dose G-CSF was considered as a potential method to improve neutrophil levels. After a discussion of risks and benefits, a trial of filgrastim was agreed upon. After 72 hours, the patient’s blood neutrophils increased outside the range of neutropenia. Once cleared for surgery, the patient had a successful elective hip arthroplasty. No post-operative infection was reported, and no neutropenia was noted. This presents a potential application of filgrastim or another low-dose G-CSF as prophylaxis to treat moderate neutropenia prior to elective surgery.
CONSENT STATEMENT
Written consent for individual health information was obtained for this publication.






