Presenter: Rachael Grace
Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, Harvard Medical School, Massachusetts, USA
Disclosure: Grace has received research funding from Agios, Dova, and Novartis; and has received consulting services from Agios and Principia.
Acknowledgements: Writing assistance was provided by Helen Boreham, HB Medical (UK) Ltd, Leeds, UK.
Support: The publication of this article was funded by Agios. The views and opinions expressed are those of the presenter.
Citation: EMJ Hematol. 2022;10[1]:21-27. DOI/10.33590/emjhematol/10057650. https://doi.org/10.33590/emjhematol/10057650.
Summary
Three posters presented at the European Hematology Association (EHA) 2022 Hybrid Congress focused on the role of mitapivat in the treatment of pyruvate kinase (PK) deficiency, a rare inherited chronic haemolytic anaemia.1-3 Mitapivat is a first-in-class, oral, allosteric activator of the red blood cell (RBC) PK enzyme, and treats the underlying pathophysiology of PK deficiency.1-3 Results from a long-term extension study in adults have confirmed the consistency and durability of treatment response achieved with mitapivat, providing the basis for its ongoing evaluation in the treatment of paediatric patients with PK deficiency.1 Currently, there are no approved pharmacotherapies that target the underlying cause of haemolytic anaemia in children with PK deficiency. To address this global unmet need, recruitment is currently underway into two international Phase III paediatric studies (ACTIVATE-KidsT [NCT05144256]4 and ACTIVATE-Kids [NCT05175105]5), which will evaluate efficacy and safety of mitapivat treatment in children with PK deficiency who are regularly transfused and not regularly transfused, respectively.2,3Durability of Haemoglobin Response and Reduction in Transfusion Burden is Maintained Over Time in Patients with Pyruvate Kinase Deficiency Treated with Mitapivat in a Long-Term Extension Study
Rachael Grace
PK deficiency is a rare, life-long, hereditary disorder caused by mutations in the PKLR gene, which encodes for the RBC-specific PK enzyme.6,7 Defects in this critical enzyme lead to chronic haemolytic anaemia.6,7 Patients with PK deficiency are at risk of both acute and long-term disease-related complications, independent of their transfusion needs, including iron overload, pulmonary hypertension, cholelithiasis, and osteoporosis.8-11 The disease also exerts a substantial negative impact on patients’ health-related quality of life.10
Mitapivat is an oral allosteric activator of PK that is approved by the U.S. Food and Drug Administration (FDA) for the treatment of haemolytic anaemia in adults with PK deficiency, and has been submitted for regulatory review to the European Medicines Agency (EMA).12-14 FDA approval was based on positive findings from two global, Phase III, clinical trials (ACTIVATE, placebo controlled [NCT03548220]15 and ACTIVATE-T open label [NCT03559699]16), both of which met their primary efficacy endpoint.17,18
In the ACTIVATE study of patients with PK deficiency who were not regularly transfused, mitapivat demonstrated a significantly higher haemoglobin (Hb) response rate than placebo (40% versus 0%; 2-sided p<0.001, respectively).17 Improvements in markers of haemolysis and haematopoiesis were also seen, together with an improvement in PK deficiency-specific measures of health-related quality of life.17
In the ACTIVATE-T study of patients with PK deficiency who were regularly transfused, 37% of patients (n=10) achieved a reduction in transfusion burden (1-sided p=0.0002, respectively).18 Mitapivat proved well-tolerated across both Phase III studies, with a consistent safety profile.17,18
This poster, by Grace et al.,1 presented at EHA2022, showcased data from ACTIVATE, ACTIVATE-T, and their long-term extension (LTE) study (NCT03853798).19 Patients who completed the fixed-dose period in both studies and demonstrated clinical benefit from mitapivat treatment, or were assigned to placebo were eligible to continue in the LTE, where all received mitapivat. The ACTIVATE/LTE analysis assessed duration of Hb response in two cohorts: patients assigned to mitapivat who achieved a Hb response and continued to the LTE (mitapivat-to-mitapivat arm); and patients assigned to placebo who switched to mitapivat in the LTE placebo-to-mitapivat arm) and then met Hb response criteria. The ACTIVATE-T/LTE analysis assessed transfusion response in the LTE, and transfusion-free duration among patients from ACTIVATE-T who achieved transfusion-free status.1
In the ACTIVATE/LTE study, patients who were not regularly transfused and randomised to mitapivat showed maintenance of their Hb response for 72 weeks.1 In total, 13 out of 15 (86.7%) patients who achieved an initial Hb response to mitapivat in ACTIVATE and were evaluable for Hb assessment in LTE sustained those increases in Hb concentration above the response threshold of ≥1.5 g/dL for a period up to 19.5 months.1 Similarly, six of 17 ACTIVATE patients who had sufficient time in LTE for Hb assessment (35%) who switched from placebo to mitapivat achieved a Hb response which was maintained for the duration of follow-up.1
In the ACTIVATE-T/LTE study, transfusion reduction response and extended duration of transfusion-free status was attained in patients who were regularly transfused. Nine patients (33.3%) in the LTE met criteria for a transfusion response. All six patients who were regularly transfused, and who achieved transfusion-free status during treatment with mitapivat in ACTIVATE-T, maintained this status through the LTE for up to 21.9 months.1
Collectively, results from the LTE study show the sustained efficacy of mitapivat in improving Hb and reducing transfusion burden in patients with PK deficiency over time.1 The safety and long-term durability of treatment response with mitapivat support its evaluation in children with PK deficiency.2,3
ACTIVATE-KidsT: Mitapivat in Children with Pyruvate Kinase Deficiency Who Are Regularly Transfused
Rachael Grace
In the absence of any approved pharmacotherapy for PK deficiency in children, RBC transfusions remain the mainstay of disease management for children less than 5 years of age.12,20 Over half (53%) of children with PK deficiency younger than 5 years old require regular transfusions at an average interval of every 5 weeks.20 Frequent transfusions impose a heavy burden on patients and their families, and can also exacerbate complications such as iron overload.17 Splenectomy is commonly performed in children aged 5 years or older with PK deficiency in an attempt to alleviate these transfusion needs; however, the procedure itself carries lifelong risks of sepsis and thrombosis, and is only partially effective at improving anaemia.20,21
To address the clear unmet need that exists in paediatric patients with PK deficiency who are regularly transfused, recruitment is currently ongoing for ACTIVATE-KidsT, a global Phase III study of mitapivat. In this poster, Grace et al.,2 presented the study design, key efficacy endpoints, eligible patient population, and geographical locations of this landmark clinical trial.
ACTIVATE-KidsT is an international, Phase III, multicentre, randomised, double-blind, placebo-controlled study that will evaluate the efficacy and safety of mitapivat in children aged 1–<18 years of age with PK deficiency who are regularly transfused. ‘Regularly transfused’ is defined as undergoing between six and 26 transfusion episodes in the 52-week period prior to providing informed consent for the study.2 In addition to meeting the study transfusion criteria, patients must have central laboratory genotyping confirming PK deficiency, defined as ≥2 mutant alleles in the PKLR gene. Additionally, subjects must have ≥1 missense mutation, and not be homozygous for the R479H mutation or have two non-missense mutations. Key exclusion criteria for the ACTIVATE-KidsT trial include patients currently receiving haematopoietic simulating agents, or having undergone prior bone marrow or stem cell transplantation.2,3
Figure 1 shows the full study design details for the ACTIVATE-KidsT triaI. After an initial 8-week screening period, patients will enter the double-blind phase of the study, consisting of 8 weeks of individualised dose titration, followed by a 24-week fixed-dose period.2 Patients who complete the double-blind period of ACTIVATE-KidsT will also be eligible to receive ongoing mitapivat therapy for up to 5 years as part of a planned open-label extension.2
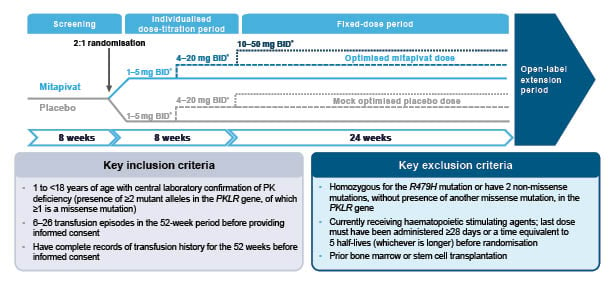
Figure 1: ACTIVATE-KidsT study design.2
*Dose of mitapivat or matched placebo based on patient’s age and weight.
BID: twice daily; Hb: haemoglobin; PK: pyruvate kinase; RBC: red blood cells.
The ACTIVATE-KidsT study aims to enrol 45 children in total, who will be stratified according to their age (1–<6 years; 6–<12 years; 12–<18 years) and splenectomy status (yes or no). A minimum of six patients in each age group will be randomised in a 2:1 ratio to receive either mitapivat or placebo at doses of 1–50 mg twice daily.2
Patients will progress through two sequential mitapivat dose increases occurring at approximately 4-week intervals in order to gradually increase Hb levels and maximise efficacy. The specific dose administered at each dose level will depend on the subject’s age and weight at the time of randomisation. The drug itself will be administered orally, either as granules or tablets.2 Paediatric dosing of mitapivat is based on pharmacokinetic modelling and simulation, aimed at reaching similar exposure to adults at the same dose level, adjusted for age and weight.22
The primary endpoint of the ACTIVATE-KidsT trial is transfusion reduction response. This is defined as a 33% or greater reduction in the total RBC transfusion volume during the fixed-dose period, normalised by weight and actual study drug duration, compared with the historical transfusion volume standardised by weight and to 24 weeks.2 The ACTIVATE-KidsT trial will also evaluate secondary and exploratory endpoints of mitapivat efficacy and safety. Secondary endpoints will explore the effect of mitapivat on transfusion-free response, Hb, haemolysis, erythropoiesis, iron metabolism and overload, safety, quality of life, and pharmacokinetics.
ACTIVATE-KidsT will be open at approximately 27 study sites globally including in the USA, Germany, Denmark, the Netherlands, the UK, Spain, Switzerland, Italy, Czechia, and Türkiye (Figure 2).
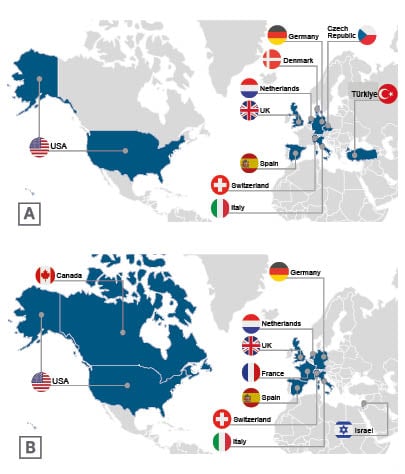
Figure 2: A) ACTIVATE-KidsT and B) ACTIVATE-Kids Phase III planned study geographic distribution.2,3
ACTIVATE-Kids: Mitapivat in Children with Pyruvate Kinase Deficiency Who Are Not Regularly Transfused
Rachael Grace
Children with PK deficiency are affected by the chronic haemolytic anaemia that is the hallmark of the disease and, regardless of transfusion status, they are at risk of developing a spectrum of lifelong disease-related complications.3 In this patient population, supportive care includes intermittent transfusions and consideration of splenectomy, both of which are associated with the potential for long-term complications.
In this poster, Grace et al.3 shared details of the ACTIVATE-Kids study, which will evaluate the efficacy and safety of mitapivat in children with PK deficiency who are not regularly transfused. Similar to ACTIVATE-KidsT, ACTIVATE-Kids is a Phase III, global, multicentre, randomised, double-blind, placebo-controlled study targeted at patients aged 1–<18 years of age with two or more PKLR mutant alleles, of which at least one is a missense mutation.3 In order to be eligible to participate in ACTIVATE-Kids, patients much have received no more than five RBC transfusions in the 52 weeks prior to informed consent, and no transfusions in the 12-week period before receiving the first dose of mitapivat.3 In addition, participants must have a Hb concentration ≤10 g/dL for patients aged 12–<18 years and ≤9 g/dL for patients aged 1–<12 years of age during screening.3 Patients must have genetic confirmation of PK deficiency and have ≥2 mutant alleles in the PKLR gene, of which ≥1 is a missense mutation. Key exclusion criteria for ACTIVATE-Kids are similar to those already described for the ACTIVATE-KidsT trial.2,3
The study design for the ACTIVATE-Kids trial is outlined in Figure 3. The screening and individualised dose-titration period are both 8 weeks long, followed by a 12-week fixed-dose period. In total, the double-blind period of ACTIVATE-Kids consists of an 8-week dose-titration period and a 12-week fixed-dose period. ACTIVATE-Kids will be followed by a 5-year open-label period, where all patients will be eligible to receive mitapivat.3
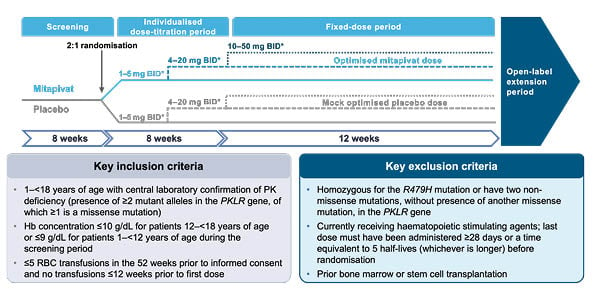
Figure 3: ACTIVATE-Kids study design.3
*Dose of mitapivat or matched placebo based on patient’s age and weight.
BID: twice daily; Hb: haemoglobin; PK: pyruvate kinase; RBC: red blood cells.
ACTIVATE-Kids aims to recruit 30 children with PK deficiency who are not regularly transfused. Patients will be stratified by age (1–<6 years; 6–<12 years; 12–<18 years) and at least six patients in each age group will be randomised 2:1 to treatment with either mitapivat or placebo, dosed at 1–50 mg twice daily. The mitapivat dose-titration and general dosing strategy will be the same as those described for the ACTIVATE-KidsT trial.2 Dosing will be tailored according to patients’ age and weight, and based on pharmacokinetic modelling of adult patients with PK deficiency.3,20
The primary endpoint of the ACTIVATE-Kids trial is Hb response defined as an increase of at least 1.5 g/dL in Hb concentration from baseline that is sustained at two or more scheduled assessments at Weeks 12, 16, and 20 in the double-blind period. Patients’ Hb concentration at baseline is defined as the average of all available Hb concentrations collected for that patient during the screening period up to administration of the first dose of study drug.3 Secondary endpoints will also be assessed during the ACTIVATE-Kids trial, and include the effect of mitapivat on haemoglobin, haemolysis, erythropoiesis, iron metabolism and overload, safety, quality of life, and pharmacokinetics.3
ACTIVATE-Kids will be a global trial, recruiting patients from approximately 20 study sites in the USA, Canada, Europe, and Israel. Participating centres in Europe are located in Germany, the Netherlands, the UK, France, Spain, Switzerland, and Italy (Figure 2).3
Conclusion
PK deficiency is a disease where unmet need remains high in children and therapies targeting the underlying cause of haemolysis are urgently needed. Long-term follow-up of Phase III trials of mitapivat in adults shows a durable Hb improvement and reduced transfusion burden. Mitapivat also proved well-tolerated in these clinical studies, with a consistent safety profile. These results support its evaluation in paediatric patients with PK deficiency. Global site recruitment is currently in progress into two Phase III trials (ACTIVATE-KidsT and ACTIVATE-Kids) of mitapivat treatment in paediatric patients with PK deficiency.







