Abstract
von Willebrand factor (VWF) is a large, adhesive, multimeric protein involved in haemostasis. The larger the size (or number of VWF multimers), the greater the functionality of the protein. A deficiency or defect in VWF can lead to von Willebrand disease (VWD) and cause bleeding, whereas an increase in VWF may cause thrombosis. ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), sometimes called VWF-cleaving protease, is primarily responsible for controlling the size of VWF. Although a deficiency of ADAMTS13 may be caused by many factors, the most severe deficiency (<10% of normal levels) arises in thrombotic thrombocytopenic purpura, which is characterised by the presence of ultra-large VWF and resulting thrombosis. The relative levels of both VWF and ADAMTS13 can be described by either the VWF/ADAMTS13 ratio or the ADAMTS13/VWF ratio, depending on author preference. Typically, this reflects VWF antigen levels and ADAMTS13 activity. The normal ratio, where both VWF and ADAMTS13 are in balance, is close to unity (or 1.0). In VWD, the VWF/ADAMTS13 ratio approaches zero, whereas in thrombotic thrombocytopenic purpura the ADAMTS13/VWF ratio approaches zero. Recent evidence has emerged that COVID-19, which may be accompanied by high prothrombotic risk, could be characterised in some patients as expressing an imbalance of VWF and ADAMTS13, or a high VWF/ADAMTS13 ratio, in a clinical picture resembling a secondary thrombotic microangiopathy. The current narrative review discusses the so-called VWF/ADAMTS13 axis in COVID-19 and beyond.
INTRODUCTION
von Willebrand factor (VWF) is a large, adhesive, multimeric protein involved in haemostasis. The larger the size (or number of VWF multimers), the greater the functionality of the protein.1 A deficiency or defect in VWF can lead to von Willebrand disease (VWD) and cause bleeding.2 Deficiency infers a relative lack of VWF protein, whereas defect infers a dysfunctional protein.
Of more relevance to this review is that an increase in VWF may cause thrombosis.3 Although VWF increase per se may be associated with thrombosis, it is the increase in the larger VWF protein moieties (sometimes called high-molecular-weight [HMW] VWF) that are more likely to lead to thrombosis. Another protein, ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), is primarily responsible for controlling the size of plasma VWF. ADAMTS13 achieves this by proteolytic cleavage of VWF multimers and is hence sometimes called von Willebrand factor-cleaving protease.4 This knowledge developed from the pioneering work of Moake et al.5 in 1982 and Furlan et al.6 and Tsai et al.7 in 1996.
A deficiency of ADAMTS13 may be caused by many factors. Notably, the most severe deficiency of ADAMTS13 arises in thrombotic thrombocytopenic purpura (TTP).4 In addition to characteristic features, including low platelet count, microangiopathic haemolytic anaemia, and increased lactate dehydrogenase, the disorder is characterised by levels of ADAMTS13 <10% of normal, with consequent presence of ultra-large VWF; accordingly, thrombosis is a key feature of TTP.4
The relationship between VWF and ADAMTS13 is sometimes called the VWF/ADAMTS13 axis. The relative levels of both VWF and ADAMTS13 in the normal state can be described as a percentage of normal or in U/dL, which for both would average approximately 100%. The relative level or relationship can also be described by using either the VWF/ADAMTS13 ratio or the ADAMTS13/VWF ratio, depending on an author’s preference. In general, the VWF level usually used is that measured by its antigen, and the level of ADAMTS13 usually used is that measured by its activity; however, sometimes VWF activity and ADAMTS13 antigen may be used instead. Why some authors use VWF/ADAMTS13 and others ADAMTS13/VWF may relate to their main interest; those with primary interest in VWF may favour the VWF/ADAMTS13 ratio, whereas those with primary interest in ADAMTS13 may favour the ADAMTS13/VWF ratio. Preference may also relate to pragmatism. In this review, the authors use the VWF/ADAMTS13 ratio because it is the excess of VWF that essentially drives the pathophysiology of thrombosis. The normal ratio, where both VWF and ADAMTS13 are in balance, is close to unity (1.0) but may range from approximately 0.5–2.0. In either case, haemostasis related to the VWF/ADAMTS13 axis is normal or in balance (Figure 1A).
In VWD, the VWF/ADAMTS13 ratio approaches zero. Use of the alternate ADAMTS13/VWF ratio can lead to huge numbers, or even attempted numerical division by zero in severe Type 3 VWD, and therefore approaches infinity. In contrast, in thrombotic microangiopathies the ADAMTS13/VWF ratio approaches zero; alternate use of the VWF/ADAMTS13 ratio can lead to huge numbers, or even attempted division by zero in TTP, the most severe microangiopathy, and thus approaches infinity. In either case (i.e., for VWD or TTP), neither ratio (VWF/ADAMTS13 or ADAMTS13/VWF) is usually reported for reasons explained below. In the authors’ view, this is appropriate. Nevertheless, the ratio between VWF and ADAMTS13 has been shown to be of clinical significance in several pathological conditions; for example, as a predictor of outcome in acute ischaemic brain injury,8 predicting thrombotic complications in cirrhosis and post-hepatectomy,9,10 and prognostication after myocardial infarction.11
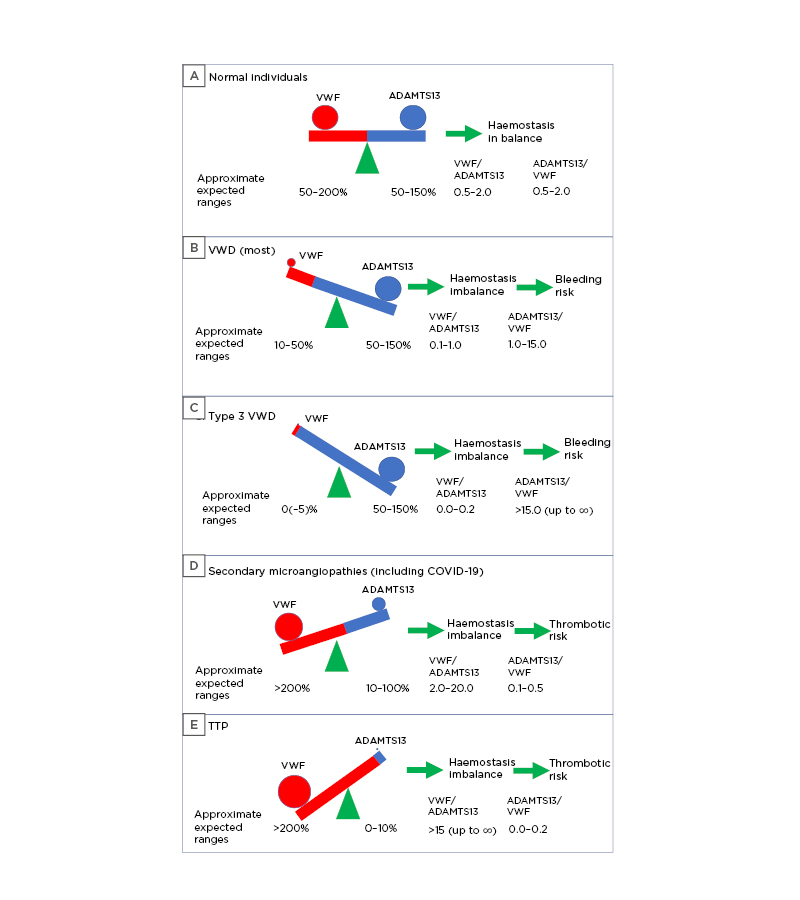
Figure 1: The VWF/ADAMTS13 axis in A) normal individuals; B and C) VWD; and D and E) microangiopathies.
COVID-19 may present as a D) secondary thrombotic microangiopathy. The stated values of VWF, ADAMTS13, and their ratios are approximate only. Values differ according to VWF and ADAMTS13 test methods, and according to patient status.
ADAMTS13: a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; VWD: von Willebrand disease; VWF: von Willebrand factor; TTP: thrombotic thrombocytopenic purpura.
More relevant for this review is that recent evidence has emerged suggesting COVID-19, which is often associated with a high prothrombotic risk,12-15 may be characterised in some patients as an imbalance of VWF and ADAMTS13, or a high VWF/ADAMTS13 ratio, in a clinical picture closely resembling a secondary thrombotic microangiopathy. The current narrative review discusses the so-called VWF/ADAMTS13 axis in COVID-19 and beyond. The authors also advocate for increased use of VWF/ADAMTS13 reporting in a wide variety of pathological conditions involving their dysregulation.
VON WILLEBRAND FACTOR AND DISEASE
As mentioned, VWD is characterised by low levels of or defective VWF;2 this causes bleeding. In these patients, the focus is on VWF because this represents the abnormal event.
There are three main types of VWD. Types 1 and 3 represent partial and total deficiencies in VWF, respectively, and Type 2 comprises four separate subtypes: 2A, which represents selective deficiency in HMW VWF; 2B, which represents a hyperfunctional form of VWF, the consequence of which is also deficiency in HMW VWF; 2N, which represents a relative defect in VWF binding to factor VIII, the consequence of which is reduced plasma levels of factor VIII; and 2M, which represents a relative defect in VWF not characterised by the other types (Table 1).2,16 Of interest, the clinical severity of VWD is not always proportional to the level of VWF but in general, the lower the level of VWF activity, the greater the bleeding risk.2
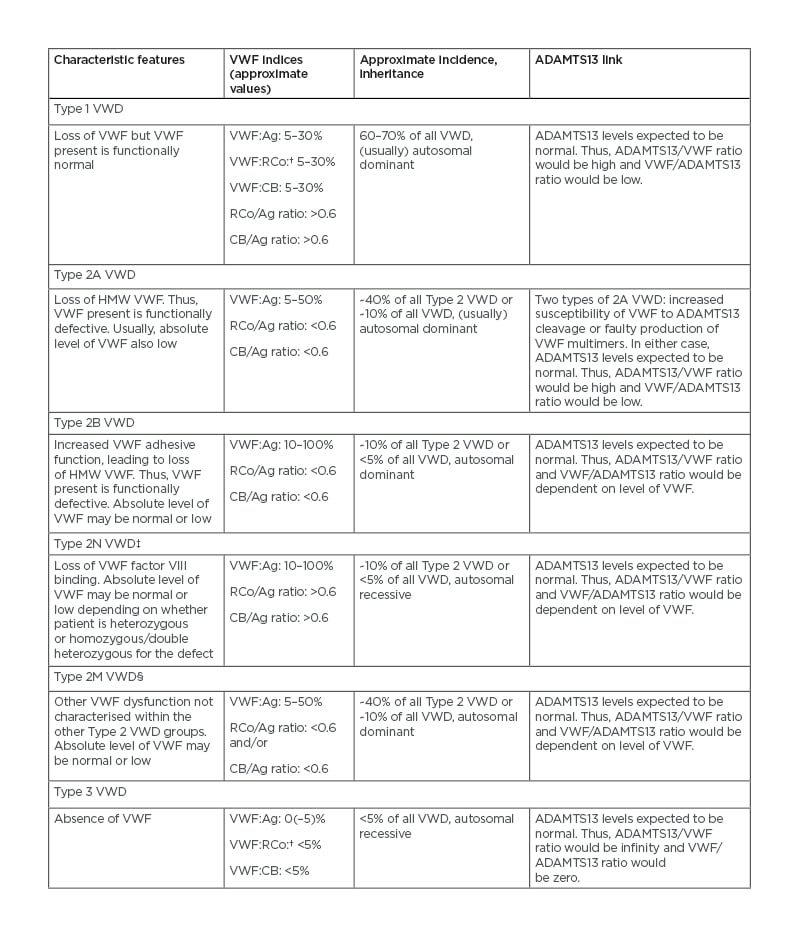
Table 1: Types of von Willebrand disease.*
*ADAMTS13 levels would be expected to be normal in patients with VWD; however, ADAMTS13 levels are not normally assessed in these patients. This is because the important findings relate to levels and function of VWF. Loss of VWF or VWF function represents a risk factor for bleeding.
†VWF:RCo represents an original platelet GPIb binding assay that uses ristocetin and platelets to assess VWF activity. More recent variants of GPIb binding are VWF:GPIbR and VWF:GPIbM. In general, VWF:RCo, VWF:GPIbR, and VWF:GPIbM are similar in that they all assess platelet GPIb binding activity.16
‡Type 2N VWD requires other assays for diagnosis.
§Type 2M VWD may express abnormal glycoprotein Ib binding and/or collagen binding.
ADAMTS13: a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; Ag: antigen; CB: collagen binding; GPIb: glycoprotein Ib; HMW VWF: high-molecular-weight von Willebrand factor; RCo: ristocetin cofactor; VWD: von Willebrand disease; VWF: von Willebrand factor; VWF:Ag: von Willebrand factor antigen; VWF:CB: von Willebrand factor collagen binding; VWF:RCo: von Willebrand factor ristocetin cofactor.
In theory, ADAMTS13 levels should be normal in VWD but are not generally assessed. This is entirely appropriate because the pathological state is bleeding caused by a lack of or dysfunctional VWF. Consequently, there is no anticipated change in ADAMTS13 level or activity. Thus, clinicians may see results related to VWF level (antigen; VWF:Ag) and function (e.g., platelet binding or collagen binding) as relevant but not results related to ADAMTS13.2,16 Thus, neither VWF/ADAMTS13 nor ADAMTS13/VWF ratios are ever reported in VWD. This is of course appropriate. However, should the VWF/ADAMTS13 axis be considered this could be determined to be in an imbalanced state because of the reduction in either VWF level or activity (Figures 1B and C).
Of additional interest is that Type 2A VWD may arise from either decreased production of HMW VWF caused by faulty assembly of HMW multimers or increased clearance of HMW VWF by ADAMTS13 as a result of structural changes in VWF, making this dysfunctional VWF more susceptible to cleavage by ADAMTS13.
ADAMTS13 IN THROMBOTIC THROMBOCYTOPENIC PURPURA AND OTHER THROMBOTIC MICROANGIOPATHIES
As mentioned, TTP is characterised by the deficiency of ADAMTS13, typically <10% of normal activity.4 A functional or quantitative deficiency in ADAMTS13 can lead to microvascular thrombosis because of accumulation of ultra-large VWF multimers, which would normally be cleaved by ADAMTS13 into smaller, less active forms to inhibit thrombosis and maintain normal haemostasis. TTP can be congenital (caused by genetic mutations) or acquired (arising from the presence of inhibitors), both resulting in microvascular thrombosis and subsequent microangiopathic haemolytic anaemia.17 The diagnostic detection of an inhibitory autoantibody (usually IgG) against the spacer domain of the protease is key to differentiating congenital from acquired TTP.17
In patients with TTP, the focus may be on both ADAMTS13 and VWF because both may represent an abnormal event, with abnormally low ADAMTS13 activity leading to abnormally high levels of ultra-large VWF.
Nevertheless, only ADAMTS13 levels are typically measured in TTP; HMW VWF levels are assumed to be high and a feature of the pathophysiology, and are thus rarely quantified. Again, neither VWF/ADAMTS13 nor ADAMTS13/VWF ratios are generally reported in TTP. The authors believe this to be appropriate for TTP, which represents an extreme version of thrombotic microangiopathy. However, should the VWF/ADAMTS13 axis be considered this could be determined to be in an imbalanced state (Figures 1D and E[hl/]) and in the opposite direction to VWD.
While the extreme deficit of ADAMTS13 activity (<10%) is pathognomonic for TTP, substantial decreases in ADAMTS13 activity have also been observed in several other thrombotic microangiopathies. Mild decreases or impairment of ADAMTS13 activity can be observed in some cases of Shiga toxin-producing Escherichia coli-associated haemolytic uremic syndrome, in which the toxin can inactivate ADAMTS13 and propagate thrombosis,18 as well as atypical haemolytic uremic syndrome, usually driven by genetically predisposed overactivation of the complement system.19 Moreover, decreased ADAMTS13 can be observed in sepsis, contributing to the septic coagulopathy and associated with poor prognosis.20 Decreases in ADAMTS13 have also been reported in patients with malignant hypertension, solid organ transplantation, malignancy, and autoimmune diseases.21
COVID-19, VON WILLEBRAND FACTOR, AND ADAMTS13
COVID-19 is caused by viral infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The predominant pathophysiological features of severe COVID-19 often reported include respiratory distress and hypoxia, further developing into severe acute respiratory syndrome (SARS), interstitial pneumonia, acute respiratory distress syndrome (ARDS), and finally multi-organ injury.12 Lung involvement largely stems from high expression of angiotensin-converting enzyme 2 receptors (ACE2R) on endothelial cells, which constitute almost one-third of the cells in the alveolar component of the lungs.13 ACE2R constitute a binding site for SARS-CoV-2 and probably the main entry point for viral invasion. In the lungs, this leads to endothelial cell activation followed by an inflammatory–thrombotic process and an associated coagulopathy.12 However, it is now also clear that both localised microthrombosis and peripheral or systemic thrombosis may develop. Thus, both pulmonary thrombosis and pulmonary embolism (PE) can occur, along with micro- and macrothrombosis in other organs (e.g., heart, kidney, liver, and spleen).14 Essentially, any tissue or organ containing endothelium or otherwise expressing significant numbers of ACE2R can be the target of COVID-19-associated coagulopathy. Indeed, COVID-19 affects all facets of haemostasis, including endothelium, platelets, coagulation, and fibrinolysis.12-15,22,23 COVID-19 can therefore cause a variety of pathologies depending on which organ is damaged, especially cardiac damage and acute kidney injury (AKI). Similarly, it may be difficult to tease out microthrombosis in these patients, which is typically only seen on post-mortem analysis.24
Of particular relevance to the current review is that several reports have now identified increased levels of VWF and/or decreased levels of ADAMTS13 to be associated with COVID-19 and the severity of COVID-19 or associated multi-organ injury ([hl]Table 2).25-60 In this regard, COVID-19 may represent a clinical picture similar to a secondary thrombotic microangiopathy (Figure 1D).
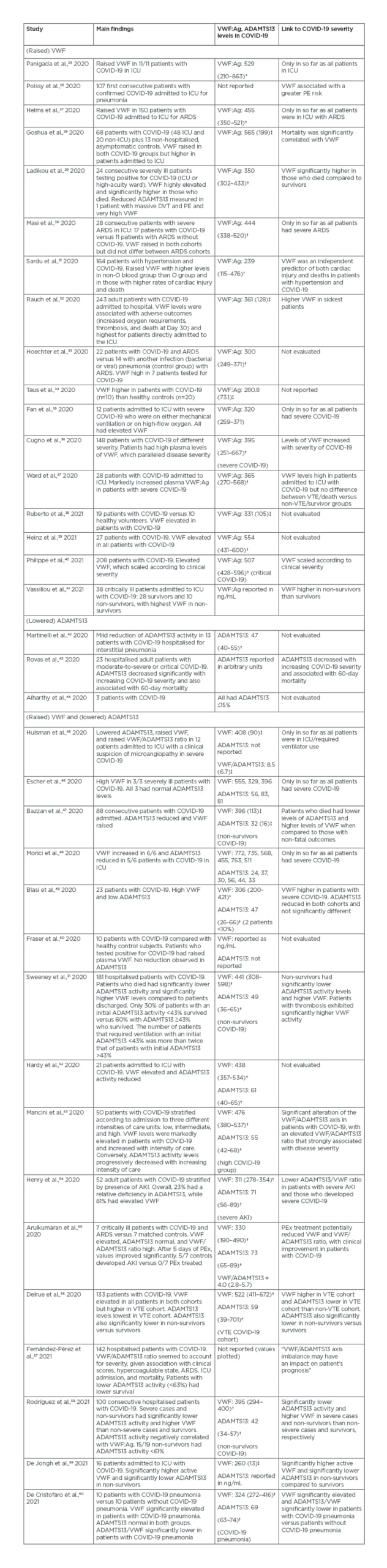
Table 2: Summary of literature related to VWF and ADAMTS13 in COVID-19.
*Mean (minimum–maximum).
†Median (interquartile range).
‡Mean (± standard deviation).
Numerical values are given in U/dL (= % of normal).
ADAMTS13: a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; AKI: acute kidney injury; ARDS: acute respiratory distress syndrome; DVT: deep vein thrombosis; ICU: intensive care unit; PE: pulmonary embolism; PEx: plasma exchange; VTE: venous thromboembolism; VWF: von Willebrand factor; VWF:Ag: von Willebrand factor antigen.
Literature Review
A PubMed search of ([“VWF” OR “ADAMTS13”]AND “COVID-19”), using various configurations of these search words, identified 77 reports as at 30th January 2021. No language restrictions were imposed but all retrieved reports were in English. The reports were screened by the lead author using the title and abstract or, in the case of no abstract, by review of the full citation. After exclusion of irrelevant papers (i.e., those not reporting on VWF or ADAMTS13 in COVID-19), reviews and commentaries without any original data on VWF or ADAMTS13 in COVID-19, and single-case studies, 17 papers were identified that reported on VWF in COVID-19,25-41 3 that reported on ADAMTS13 and COVID-19,42-44 and 16 that reported on both VWF and ADAMTS13 in COVID-19.45-60
Results of the Literature Review
The results of the literature review are summarised in Table 2. A unifying theme in the reporting papers was elevation of VWF levels and activity in patients with severe COVID-19. That is, all papers identifying VWF levels (antigen or activity) in patients with COVID-19 reported elevated VWF, with levels higher than 600 U/dL in some cohorts, as opposed to normal reference ranges approximating 50–200 U/dL, thus representing a 2–6-fold increase over normal levels. In addition, some reports also identified an association between VWF level and severity of COVID-19, including VWF being higher in non-survivors as opposed to survivors, higher in those admitted to intensive care units as opposed to general wards, and higher in those with greater oxygen requirements (i.e., high-flow oxygen or invasive ventilation). Findings were mixed regarding the linkage of VWF to thrombosis risk per se. For example, Poissy et al.26 reported that higher VWF:Ag was associated with a greater PE risk, Sweeney et al.51 reported that patients with thrombosis exhibited significantly higher VWF activity, and Delrue et al.56 reported higher VWF:Ag in those with venous thromboembolism. In contrast, both Rauch et al.32 and Ward et al.37 reported that the association of VWF:Ag to thrombosis was non-significant. These heterogenous findings may be attributable to the type of thrombosis as well as the inflammatory state, which is always a confounder in VWF measurements. Thus, VWF is a well-recognised acute phase reactant and is increased in a variety of inflammatory states.61 On the other hand, VWF is not the only acute phase protein; factor VIII and fibrinogen both increase acutely following viral infections, including COVID-19, and may thus also fuel prothrombotic outcomes.62 Additional explanations for these heterogenous findings include the composition, number, and pathophysiological status of the COVID-19 population cohort under investigation, which was broad in terms of included cases. Most of the studies assessing thrombosis were based on venous thromboembolism, as potentially reflected by PE or deep vein thrombosis. These types of thromboses may become evident by standard clinical investigation such as (Doppler) ultrasound, plethysmography, angiography, lung ventilation scan, or CT scan. However, it is the formation of microthrombosis that elevated VWF may be preferentially causing, as may be anticipated in thrombotic microangiopathies. Microthrombi are more difficult to assess clinically and instead may only be evident in autopsy reports.12,14,15,24
Where ADAMTS13 was evaluated in patients with COVID-19, this was either found to be at normal levels or reduced. In some cases, levels were low enough to be consistent with a secondary thrombotic microangiopathy, including a TTP-like syndrome, with ADAMTS13 levels occasionally reaching <10%. Where antibodies to ADAMTS13 were evaluated, these were generally not found. Thus, the picture essentially reflects an ADAMTS13 consumption event, likely associated to the level of VWF and presence of endothelial activation rather than an autoimmune picture like in acquired TTP.4
Overall, VWF elevation has been more consistently observed in the literature than has a reduction in ADAMTS13 (Table 2). However, looking at VWF or ADAMTS13 alone, without consideration of the other, could lead to misrepresentative findings and explain some of the differences observed between studies. Indeed, a significant increase in VWF level alone (without any evident reduction in ADAMTS13 level) would still lead to an imbalance of the VWF/ADAMTS13 axis and ultimately infer a prothrombotic risk (Figure 1D). Although ADAMTS13 may be in the normal range (approximating 50–150 U/dL), it is likely that a reduction may still be occurring in many of these patients relative to their own baseline (pre-COVID-19) levels of ADAMTS13.
Many investigations formally looking at the VWF/ADAMTS13 axis in COVID-19 have reported an association with worse outcomes. For example, Bazzan et al.47 found that patients with COVID-19 who died had significantly lower levels of ADAMTS13 and higher levels of VWF compared to those with non-fatal outcomes. Sweeney et al.51 also reported that non-survivors had significantly lower ADAMTS13 activity levels and higher VWF, with ADAMTS13 activity inversely correlated with VWF. Additionally, the number of patients requiring ventilation with initial ADAMTS13 <43% was more than twice that of those with initial ADAMTS13 above this threshold.51 Mancini et al.53 also reported that the VWF/ADAMTS13 ratio was associated with COVID-19 disease severity (medians of 3.42, 6.77, and 8.33 in low, medium, and high severity, respectively). Rodríguez et al.58 reported significantly lower ADAMTS13 activity and higher VWF (thus, elevated VWF/ADAMTS13) in severe cases and non-survivors as compared to non-severe cases and survivors, respectively. De Jongh et al.59 similarly reported significantly higher active VWF and significantly lower ADAMTS13 in non-survivors versus survivors. Furthermore, De Cristofaro et al.60 reported that VWF was significantly elevated and ADAMTS13/VWF significantly lower (i.e., VWF/ADAMTS13 higher) in patients with COVID-19 pneumonia versus patients without COVID-19 pneumonia. Finally, Henry et al. reported a lower ADAMTS13/VWF ratio (equivalent to a higher VWF/ADAMTS13 ratio) in those who developed severe COVID-19 and in those who developed severe AKI, a hallmark of thrombotic microangiopathies. 54
Further research is still needed to fully understand the mechanisms associated with the prothrombotic state in COVID-19. This includes studying how the VWF/ADAMTS13 axis imbalance is connected to the intermingled mechanisms of SARS-CoV-2 pathophysiology, such as immune dysregulation, complement overactivation, neutrophil extracellular traps, and auto-antibodies, which may all converge to propagate COVID-19-associated coagulopathy.12
There were several striking disparities in terms of data reporting that complicated assessment of the commutability between studies. Firstly, although VWF was usually reported as the level of antigen and ADAMTS13 as the level of activity, sometimes authors instead reported VWF activity and ADAMTS13 antigen. Secondly, although VWF:Ag and ADAMTS13 activity were most often reported, these tests were performed using a wide range of methodologies. For instance, VWF:Ag was assessed using both commercial and in-house enzyme-linked immunosorbent assays, commercial latex immunoassays, and chemiluminescence immunoassays. These utilise different capture and detection antibodies and different assay calibrators, thus affecting both normal reference range data as well as arising values in patients with COVID-19. When reported, VWF activity was even more varied and included ristocetin cofactor assays or other platelet glycoprotein Ib (GPIb) binding assays, such as VWF:GPIbR (recombinant) or VWF:GPIbM (mutant). Collagen binding activity was rarely reported. ADAMTS13 activity was measured using in-house or commercial fluorescence resonance energy transfer methods and chemiluminescence immunoassays. Sometimes, methods used were not described at all, which made comparisons between studies essentially impossible.
Despite discussing the imbalance between VWF and ADAMTS13 in COVID-19, very few reports provided values for either VWF/ADAMTS13 or ADAMTS13/VWF ratios, which the authors believe will become an important parameter in a variety of pathophysiological states, including COVID-19. The authors thus call on future researchers to report such values more widely in the future.
Lastly, the authors considered the wide variety of grades of COVID-19 severity. This condition develops throughout a vast array of clinical pictures, ranging from totally asymptomatic or paucisymptomatic (e.g., only causing influenza-like symptoms), then progressing to lower respiratory tract involvement (interstitial pneumonia), and finally evolving into a systemic disorder that may ultimately lead to multi-organ failure and death.63 Since thrombosis is a hallmark of the evolving pathology, it is likely that the degree of impairment of the VWF/ADAMTS13 axis would progress in parallel with disease worsening.
Further Therapeutic Developments
A secondary thrombotic microangiopathy requires treatment of the driving force (presumably COVID-19 in this instance). The above observations therefore pave the way to evaluating specific therapies already used in secondary microangiopathies, such as complement inhibitors (e.g., eculizumab). Although other similar agents, including ravulizumab, have not been overly successful in trials for COVID-19, preliminary data suggest that this therapeutic approach may complement others to improve survival and lower hypoxia, especially in patients with COVID-19 and severe illness or ARDS,64-66 thus suggesting that secondary thrombotic microangiopathy may be a major driver of outcomes in SARS-CoV-2 infection. The use of recombinant ADAMTS13 is another possible option to lower abnormally upregulated VWF factor in the plasma of patients with COVID-19, as recently highlighted by Turecek et al.;67 however, this would need to be tested in future trials. Also of relevance is the small study by Arulkumaran et al.,51 who utilised plasma exchange in seven critically ill patients with COVID-19 and ARDS versus seven matched controls, and who not only showed improvement in patients after plasma exchange but also noted that the majority (n=5/7) of controls developed AKI, whereas AKI was developed by none of the seven plasma-exchange-treated patients. Thus, therapies normally used in TTP may also be useful in the management of COVID-19.
CONCLUSION
In summary, recent evidence strongly suggests that COVID-19 can progress towards a thrombotic disorder, characterised by both micro- and macrothrombosis in the lungs, as well as in many other organs and tissues.12-15 The development of any form of thrombosis, which can then have a strong impact on a patient’s prognosis, appears to be accompanied by an imbalance of VWF and ADAMTS13, evidenced by a high VWF/ADAMTS13 ratio, thus generating a clinical picture that closely resembles a secondary thrombotic microangiopathy. The authors highly recommend reporting of VWF/ADAMTS13 ratios in future COVID-19 studies to give additional context to the altered VWF/ADAMTS13 axis in these patients.







