Abstract
In 2016, an update on the classification of lymphoid neoplasm was published, and one of the modifications made focussed on B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt’s lymphoma, a term which has now been abandoned. This represented a very difficult disease in the diagnostic routine of pathologists. The new proposed term is high-grade B-cell lymphoma, which includes the double-hit lymphomas. Yet, there was still confusion about the diagnostic criteria. This review discusses the changes in classification, with an emphasis on the double-hit lymphomas. Diffuse large B-cell lymphoma and Burkitt’s lymphoma are also commented on in the text. The diagnosis of double-hit lymphomas is dependent on molecular tests and it is not available throughout the world. Research identifying features that can allow patients to be specifically selected for these molecular tests is also important.
INTRODUCTION
Lymphoma is a malignant neoplasm characterised by clonal proliferation of B lymphocytes, T lymphocytes, or natural killer cells (mature or immature), at different stages of differentiation.1 Classically, there are two major groups of lymphomas: Hodgkin (HL) and non-Hodgkin (NHL), due to morphological and clinical differences.2,3 The main NHL is diffuse large B-cell lymphoma (DLBCL).4-7
The Globocan project, conducted by the International Agency for Research on Cancer (IARC) in 184 countries in 2012, revealed that the incidence of NHL worldwide was estimated to be 385,741 cases, with ˜199,000 deaths due to the disease.8 Over the last 25 years, there has been an increase in the worldwide incidence rates of NHL. People ageing and the increase in the number of immunocompromised individuals, for example those infected with human immunodeficiency virus (HIV), may have contributed to this epidemiological scenario.9
The last edition of World Health Organization (WHO) Classification of Tumours of Hematopoietic and Lymphoid Tissues was in 200810 and suggested the diagnostic criteria for lymphomas based on clinical, morphological, and immunophenotypic fundamentals are increasingly encompassing new frontiers of knowledge, as represented by molecular genetic studies. In the last few years, this broad set of diagnostic tools has allowed the increased understanding of the inherent complexity of this group of haematological neoplasms. This complexity is demonstrated by the mimicry of morphological patterns, by the possibilities of transformation leading to greater aggressiveness, or by the existence of tumour sub-clones coexisting with the main clones.11
NHLs are a heterogeneous group of malignancies originating in lymphoid tissue, caused by genetic alterations that occur during the process of lymphocyte differentiation leading to the uncontrolled proliferation of a neoplastic clone.1 The process of differentiation of B lymphocytes, initiated in the bone marrow and consolidated in the lymph-nodal structures, is very dynamic and encompasses proliferative, recombinant, and differentiation stages.12 This environment is conducive to the genesis of anomalies in the expression of certain oncogenes related to cell proliferation, differentiation, and immortalisation. Depending on the stage of the maturation cycle of the lymphocyte, the changes can lead to distinct neoplastic patterns that determine the subtypes of lymphomas.7
The 2008 classification consolidated the advances of the last 20 years and reinforced the need for investigation into certain subgroups of lymphomas. Firstly, B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt’s lymphoma (B-UNC/DLBCL/BL). This name was adopted for neoplasms that present overlapping of clinicopathologic and immunophenotypic patterns of DLBCL and BL. In previous classifications, they are called Burkitt-like lymphomas. These lymphomas represent at least two situations: firstly, tumours with morphological features of BL without the typical immune-phenotype and/or cytogenetic findings, and tumours with classical aspects of DLBCL with areas of BL morphology. Secondly, there is the B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and classical HL (B-UNC/DLBCL/cHL), a group of tumours that, generally, present as anterior mediastinal mass with neoplastic cells stains for B-cell markers, CD15, and CD30. Due to the overlapping of patterns, WHO-2008 named them as ‘grey zone’ lymphomas. However, in the 2016 update, there are classification modifications in B-UNC/DLBCL/BL which will be discussed in this review. B-UNC/DLBCL/cHL, on the other hand, in the 2016 update, was fortified as a specific neoplasm group.3,5,13-17
Approximately 15% of the provisional entity B-UNC/DLBCL/BL represent the double-hit lymphomas (DHL), aggressive neoplasms presenting translocation phenomenon involving MYC combined with another event involving translocation of BCL2 or BCL6. There are no specific treatment options for B-UNC/DLBCL/BL or BL.16
This review intends to discuss the changes in the classification with an emphasis on the DHL, a subset of lymphomas that was included by the B-UNC/DLBCL/BL group but now has specific recognition criteria.
DIFFUSE LARGE B-CELL LYMPHOMA
The most common subtype of NHL in the adult population in developing and emerging countries is DLBCL, corresponding to 30–40% of cases.6,7 A portion of DLBCL cases originate from the phenomenon of transformation, in which a low-grade lymphoma, such as follicular lymphoma, undergoes new molecular events, culminating in a neoplasm of higher histological grade.18,19
DLBCL is the neoplasm of large B-lymphocytes in diffuse growth pattern, with nuclear size equal to, or exceeding, the size of the nucleus of a histiocyte, or with nuclear size twice the size of the nucleus of a lymphocyte.20 This diffuse growth pattern, together with large neoplastic lymphocytes, called centroblasts and immunoblasts, is the morphological criteria of DLBCL (Figure 1).4,5 The immunophenotypic profile of DLBCL is characterised by the expression of CD20 and associated with the variable positivity of the CD10, BCL6, and BCL2 markers, with a high cell proliferation index (50–80%).21
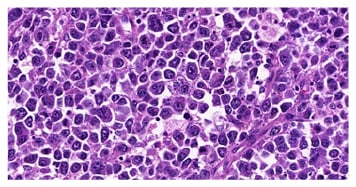
Figure 1: Morphological criteria for diffuse large B-cell lymphoma with a diffuse growth pattern and large neoplastic lymphocytes.
In the 2016 WHO update, DLBCL was subdivided into the following clinical-pathological-immunophenotypic entities: DLBCL, not otherwise specified (NOS); T cell histiocyte-rich large B-cell lymphoma; primary DLBCL of the central nervous system (CNS); primary cutaneous DLBCL; leg type; EBV+ DLBCL NOS and EBV+ mucocutaneous ulcer; and DLBCL associated with chronic infection. In 2008, the EBV+ DLBCL NOS and EBV+ mucocutaneous ulcer were in a subset called EBV-positive DLBCL of the elderly.17 This was one of the most important modifications of 2016 update because the EBV+ DLBCL NOS and EBV+ mucocutaneous ulcers are different diseases and, in the case of EBV+ DLBCL NOS, can occur at any age. Therefore, pathologists must now use these names in their reports.
This lymphoma is considered clinically aggressive, but with new therapeutics and, in initial clinical staging, it is potentially controllable.21 However, it is known that durable remissions are achieved by 40–50% of patients.22 This statistic fostered the investigation of new prognostic factors, besides those already described for the disease, this time with a molecular scope. Based on the gene expression profiling studies, Alizadeh et al.23 defined two subgroups of DLBCL: the subgroup with a germinal centre B cell (GCB) profile originating in the B lymphocytes of the lumen of the lymph node germinal centre; and the subgroup with a post-centre germ cell profile or activated B cells (ABC), which originate in mature B lymphocytes, already with some level of involvement with plasmacytoid differentiation.23
DLBCLs classified as GCB represent 45–50% of patients, and have a good prognosis, with disease-free survival in 5 years of ˜50–60%. In addition to the gene expression analysis, chromosomal alterations were also observed. The GCB profile shows gains of 12q12; the ABC profile shows gains of 3q and 18q21–22 and losses of 6q21–22.23 Recently, Basso and Dalla-Favera12 highlighted the involvement of translocations involving MYC and BCL2 in the pathogenesis of DLBCL-GCB and the importance of the activation of the nuclear factor-κβ (NF-κβ) pathway with disruption of terminal differentiation in DLBCL-GCB.12
Hans et al.,22 in turn, proposed an immune-histochemical evaluation strategy to detect these subgroups described by the gene expression profile.22 Using the CD10, BCL6, and MUM-1 markers, the authors proposed an algorithm to define these profiles. The CGB profile was defined by positivity for CD10, or by positivity for BCL6 along with negativity for CD10 and MUM-1. The ABC profile was defined by negativity for CD10 and BCL6 or for negativity for CD10 associated with positivity for BCL6 and MUM-1.22
In addition to Hans et al.,22 other groups of authors, such as Choi et al.,24 Visco et al.,25 and Meyer et al.,26 also proposed algorithms for detection of these molecular profiles by immune-histochemical study.22,24-26 Although these studies are able to define clinical, aetiopathogenic, and prognostic profiles, the diagnostic routine has not fully incorporated these concepts, given the variability of the results observed in the different algorithm models.24-26 However, in the 2016 update, it was recommended to use these algorithms in the diagnostic routine, in particular by Hans et al.22 This recommendation was based on reports on the prognostic importance of these immunohistochemistry algorithms. Besides, immunohistochemistry represents an accessible method different from molecular techniques of gene expression.17
In addition to gene expression studies, in the last decade, the research related to the identification of the translocations involved with DLBCL has increased. More than 30% of patients with DLBCL present translocations involving BCL6, which is the most common rearrangement in this lymphoma.27-30 Translocation involving BCL2, which is fundamental in the genesis of follicular lymphoma, occurs in 20–30% of DLBCL.31,32 The rearrangements involving MYC are observed in ˜10% of the cases.3,13,27,33,34
Cabanillas,27 reviewing the biology of lymphomas, highlighted advances in the detection of these translocations. In ˜20% of the cases in which the translocation of MYC was verified, there was, simultaneously, another translocation involving BCL2 and/or BCL6. These cases had high rates of cell proliferation and were the initial stages of the studies that led to the proposition of the category called lactate dehydrogenase (LDH), which belong to the provisional group B-UNC/DLBCL/BL.27
For DLBCL, the 2016 update highlighted the importance of defining the cell origin in these cases by an algorithm such as the Hans algorithm and this must now be part of the diagnostic routine of pathologists. Haematologists will have to solicit this kind of prognostic evaluation due to new drugs, such as lenalidomide, bortezomib, carfilzomib, and ibrutinib, being developed for treatment for ABC profile of DLBCL. As such, the Hans algorithm is now a reality and something for routine practice.17
BURKITT’S LYMPHOMA
BL is the most common haematological neoplasm of childhood which is potentially curable. There are three clinical variants described. Endemic BL occurs on the African continent with a peak incidence between 4 and 7 years of age. Sporadic BL affects children and young adults, with an average age of involvement of approximately 30 years. BL associated with immunodeficiency is the variant related to HIV infection.35 The association with Epstein–Barr virus (EBV) infection is seen in the majority of patients with the endemic form. In sporadic form and in the form associated with immunodeficiency, EBV is identified in ≤30% and 40% of cases, respectively.3,36,37
The clinical presentation of the disease often occurs in extranodal sites. Bones of the orbital and mandibular regions of the face are the most reported locations in the endemic form. In sporadic form, the abdomen is a common site, especially the intestine, more specifically in the ileocecal region. The form related to immunodeficiency is differentiated by nodal predilection with a greater tendency to the bone marrow.35-37 All three forms exhibit a high risk for involvement of the CNS.35
BL morphology is characterised by the proliferation of neoplastic B-lymphocytes of medium size, with nuclei similar or smaller than those seen in the histiocytes, in monomorphic and diffuse patterns of growth, with frequent figures of mitosis. A characteristic, but not pathognomonic, aspect is the presence of apoptotic bodies determining the ‘starry sky’ aspect (Figure 2).35-37
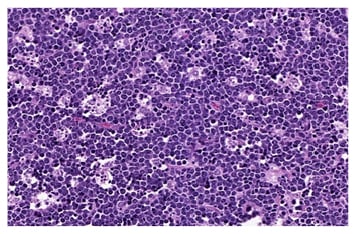
Figure 2: The ‘starry sky’ characteristic in the presence of apoptotic bodies in Burkitt’s lymphoma.
The immunophenotypic profile of LB is positivity for CD20, CD79a, PAX5, CD10, and BCL6, with negativity for BCL2 and terminal deoxynucleotidyl transferase (TDT). The cellular proliferation index of these neoplasms, evaluated by the immunohistochemical marking of Ki-67, is >90%, frequently reaching close to 100%.36,37
BL pathogenic origin is in B-lymphocytes of the dark zone of the germinal centre that aberrantly express MYC, whose translocation is visualised in 100% of the cases of this disease. In addition, in 70% of patients, the activation of the PI3K signalling pathway is achieved.12 Translocation involving MYC (8q24) occurs with one of three possible immunoglobulin (Ig) loci, being either heavy chain (IgH, 14q32) or, less common, lambda (IgL, 22q11), or kappa (IgK, 2p12). Secondary events are also described in approximately 30% of the cases: there are gains (1q, 7, 12, 13q) and deletions (6q, 13q, 17p, the latter related to TP53). Gains involving chromosomes 1 and 7 are described in 20% and 10% of the cases, respectively, presenting an association with worse prognoses.37
The endemic and sporadic forms of BL are quite aggressive but potentially curable. The use of combined chemotherapy regimens allows cure rates of around 90% when the diagnosis is made in the initial stages, and around 60–80% when the diagnosis is made in the advanced stages.35
B-CELL LYMPHOMA, UNCLASSIFIABLE, WITH FEATURES INTERMEDIATE BETWEEN DIFFUSE LARGE B CELL LYMPHOMA AND BURKITT’S LYMPHOMA
Distinguishing BL from other high-grade lymphomas with similar morphological patterns can be a challenge for pathologists, especially when it affects older individuals.33 For years, these cases have been called non-BL, atypical BL, or Burkitt-like lymphoma.3,13,33
Dave et al.38 studied eight cases of adults in which morphology and immunohistochemistry were controversial for the diagnosis of DLBCL, and that the gene profile was compatible with BL. Cytogenetic assessments of similar cases led to investigations into the detection of complex karyotypes, including multiple translocation events involving MYC and BCL2 separately. In addition, this molecular pattern was associated with worse prognosis and refractoriness to traditional therapeutic regimens.20,33,35
Based on these studies, the provisional category called B-UNC/DLBCL/BL was established to represent this group of aggressive lymphomas, with overlapping of morphological, immunophenotypic, and genetic aspects of both DLBCL and BL. Therefore, biological and clinical reasons justify the recommendation not to include these tumours in one of these two entities.37,39
The morphology of these lymphoid haematopoietic neoplasms is characterised by diffuse proliferation of medium to large cells, in the middle of scarce mature lymphocytes and little stromal reaction.
In most cases, there will be a similar appearance to that observed in LB, including the starry sky pattern. On the other hand, there are scenarios in which a greater atypia and nuclear size variation are observed, making the lesion less monotonous.40 Immunophenotypically, the neoplasms showed positivity for pan-B markers, such as CD19, CD20, CD22, and CD79a, associated with CD10 and BCL6 expression, and a high rate of cell proliferation, however, varying between 50% and 100%. The positivity for BCL2 marker, in this context, is very suggestive of this diagnosis.4,5,40
Cytogenetic studies demonstrated that a few B-UNC/DLBCL/BL patients had the combination of MYC translocation with translocations of BCL2 and/or BCL6, defining the DHL or triple-hit lymphomas (THL).1-7,16 In this context, in the 2016 update the term B-UNC/DLBCL/BL was abolished and must not be used in pathologic reports. In the following section, we will demonstrate that these neoplasms received the name high grade B-cell lymphomas (HGBL), a group of marked aggressiveness that may or may not have rearrangements involving MYC and BCL2 and/or BCL6. When the high-grade lymphomas do not present these rearrangements, they will be referred to as HGBL, NOS. However, when the high-grade lymphoma presents one, two, or three rearrangements, this must be put in their definition. For example, for a HGBL with MYC rearrangement, if two or three rearrangements were detected, they received the name DHL or THL. For a HGBL with MYC and BCL2 rearrangements, they must be classified as DHL.
DOUBLE-HIT LYMPHOMAS AND HIGH GRADE B-CELL LYMPHOMAS
A diagnosis of DHL is only determined following the results of a cytogenetic test, such as fluorescence in situ hybridisation (FISH).16 The clinical and prognostic aspects of DHL overlap with those described as B-UNC/DLBCL/BL. The biological aggressiveness of these lesions is exemplified by the greater propensity to infiltrate bone marrow and the CNS, in addition to a rather reserved prognosis characterised by a global survival of <2 years.41 DHL patients fall under other criteria of poor prognosis such as high cell proliferation, individuals >70 years of age, and clinical presentation at an advanced stage, as well as poor therapeutic response to current regimens.41-44 Figure 3 demonstrates a case of DHL.
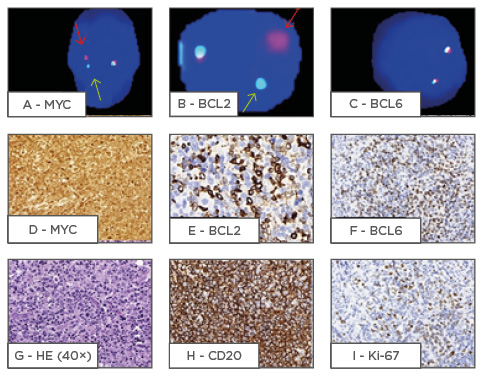
Figure 3: A demonstration of a double-hit lymphoma case.
The precise identification, which represents a challenge for pathologists because FISH exams are not available in many countries, is important for prognosis. However, the question for haematologists and pathologists is how to detect these cases. In this context, different ideas were developed to try to detect DHL by immunohistochemistry or to detect patients who really require FISH. In spite of varying reported results, there is not a standard algorithm which shows how to investigate these difficult cases.
MYC (8q24) is a transcription factor of the cell cycle, being related to mechanisms of DNA duplication/ repair, metabolic stress, and translation.13 Although its role in relation to proliferation is determined,45 its importance in the physiology of germinal centre formation is still not fully understood.12,45 It is believed that at the beginning of germinal centre reactivity, MYC induces the amplification and transcription of other genes that contribute to the proliferative processes. After the first days, this gene is inactivated by the action of BCL6, the main regulator of the germinal centre.12,45 In DBCL, its detection occurs in approximately 10% of the cases.21,46 In BL, its proliferative and apoptosis inhibitory action is observed in all patients.47
BCL6 (3q) encodes the BCL6 protein that participates in the differentiation of normal germinal centre B lymphocytes.46 This gene controls a transcriptional programme that facilitates the entry of precursor B cells into the germinal centre of the follicle. Its expression exerts modulatory and inhibitory effects on the reactions of the germinative centre, contributing to its formation and maintenance. Deregulation of this gene is associated with lymphomagenesis and is detected in 30% of DLBCL cases.16,45,47
BCL2 (18q) encodes the BCL2 protein, which promotes blockade of cellular apoptosis.7 Its translocation, frequently found in patients with follicular lymphoma, is present in 45% of cases of DLBCL and is a marker of poor prognosis.26,47,48 Recently, targeted therapies have been studied for this marker, seeking the development of drugs antagonistic to the functions of BCL2.49
DHL is the main element of the B-UNC/DLBCL/BL group.50,51 The synergistic action of the genes involved, especially MYC, with proliferative effects, and BCL2, with anti-apoptotic effects, associated with other molecular alterations may explain the biological aggressiveness of this entity.13 Aukema et al.13 proposed that aggressive B lymphomas should be investigated by cytogenetic techniques, to identify translocations involving at least MYC.13 BCL2 and BCL6 should be included in the investigation when there was immune-histochemical expression of CD10 and/or BCL6 associated with BCL2 positivity and high rates of cell proliferation.13 In selecting cases for cytogenetic investigation, Landsburg et al.52 and Green et al.53 studied the correspondence of the use of immunohistochemistry for MYC, BCL2, and BCL6 with the cytogenetic results; but had difficulty standardising percentage values to define positivity.50-53
The current parameters, whether morphological and/or immunophenotypic, are of low reproducibility and may not effectively translate the detection of the molecular alterations of MYC, BCL2, and BCL6. These translocations may also only partially represent the molecular complexity of these neoplasms.13 The three target genes, especially MYC and BCL2, share pathways related to apoptosis and cell proliferation, which are being increasingly studied with emphasis on the participation of microRNAs.13,53
In this context, Friedberg,54 at the end of a case report, whose diagnosis was DHL, questioned the need to perform the cytogenetic investigation of translocations involving MYC, BCL2, and BCL6 for all cases of DLBCL.54 There have been frequent investigations of this question. Even with the possibility of detection of these patients by immunohistochemistry.54 Zhang and Aguillera55 reviewed the application of immunohistochemistry in lymphomas and exposed the difficulties in interpreting positivity for MYC, BCL2, and BCL6, with cut-off rates varying between 40% and 50% of the cellularity expressing such antibodies.55 According to Friedberg,54 the tendency is that the search for such translocations, if possible by cytogenetic techniques, becomes routine as this information proves to be relevant for treatment and prognosis,54 which can be detailed by means of reports and investigative reviews of cases in different diagnostic centres.
Swerdlow56 emphasised the importance of identifying cases of aggressive large B cell lymphomas, mainly due to the absence of a specific therapeutic protocol.56 Such malignancies are mainly represented by DHL or THL, and their identification should be performed only by cytogenetic methods. The nomenclature of these neoplasms will be updated in the next WHO classification of haematologic malignancies.16,56 In this context, there is great interest in studies of agreement between immune-histochemical results and cytogenetic markers for MYC, BCL2, and BCL6 in the search for selector elements for molecular screening. In fact, difficulties related to the standardisation of defining criteria of positivity for MYC and BCL2 in immune-histochemical reactions has been reported.56 In general, patients with double or triple translocations identified by FISH have worse prognoses than patients with double or triple immune-histochemical positivity, which, in this study, was suggested by the proportion of deaths among different diagnostic groups.16,56 The increase in the number of articles on the subject reveals how complex the problems are, with numerous issues yet to be debated and processes that still require standardisation. Providing descriptions of these cases is, thus, all the more relevant.56 Double and triple immune-histochemical positivity in HGBL is a target of different investigators. However, this kind of expression is not compatible with DHL or THL. Pathologists must relate them in their reports but there is not a consensus about a specific new group of diseases.
In 2016, Swerdlow et al.17 published a proposal of revision in the WHO classification of 2008. These authors recognised DHL and THL as clinical and pathological categories and designated HGBL with MYC and BCL2 and/or BCL6 rearrangements. Lymphomas that potentially fall into the HGBL categories can morphologically resemble B-lymphoblastic leukaemia/lymphoma, mantle cell lymphoma, BL, and DLBCL as well as lymphomas that are intermediate between DLBCL and BL. They are lymphomas of high grade with starry sky pattern, even focal areas, or with blastoid appearance with negativity for cyclin and TDT, or which morphologically seem a BL with BCL2 immunohistochemistry positivity. There is no specific guideline to select patients which require FISH testing. Some believe that all DLBCL should have genetic studies for the detection of MYC, BCL2, and BCL6 rearrangements. Others would limit them, for example, to cases with a GCB phenotype and/or high-grade morphology or to cases with 40% MYC positive cells. In the initial immune-histochemical approach, some include the BCL2 marker, with positivity indicated by >50% of cells. MYC and/or BCL2 positivity would be a signal for recommendation of FISH.17,56 Oliveira et al.16 demonstrate that the starry sky pattern is associated with MYC rearrangements and would be a selection marker. The Ki-67 index is used to consider a prediction marker.56 Mationg-Kalaw et al.57 and Oliveira et al.16 showed that Ki-67 is not an effective predictor of the detection of cytogenetic translocations involving MYC, BCL2, and BCL6. Despite the difficulties in interpreting the immune-histochemical profiles of MYC, BCL2, and BCL6, several studies have been published on how this technique can be used as a method of screening for molecular research, since it is being incorporated into the clinical routine as this cytogenetic information becomes increasingly relevant in therapeutic practice and prognosis.16,17,56
CONCLUSION
DHL is an interesting topic of haematopathology with many unresolved issues. DHL diagnosis is dependent on FISH testing, with issues due to the technology not being available throughout the world. This review has highlighted the main terms of 2016 update which are summarised below.
i) Pathologists and haematologists must abolish the term UNC/DLBCL/BL and use the term HGBL for these cases.
ii) DLBCL were cases with classic morphology with immunoblast or centroblast cells, without starry sky pattern or atypical immune-phenotype as staining for MYC or BCL2. Pathologists must test all DLBCL for Hans algorithm marker and MYC and BCL2.
iii) The cut-offs recommended by Swerdlow et al.17 are 40% of positive cells for MYC and 50% of positive cells for BCL2.
iv) HGBL can stain for MYC and BCL2, but the FISH test can be negative. These cases are also called double expressions lymphomas. However, this is not included in the 2016 update. Pathologists may report these findings, mainly when they do not routinely use FISH. More studies are needed to determine if this is a new class of lymphomas.
v) Pathologists must consider HGBL when there is a starry sky feature, even focal, or a classical morphology of BL with BCL2 positive in immunohistochemistry or a blastoid appearance with negativity for TDT or cyclin D1. Ki67 is not a good parameter; when its level is near 100%, pathologists must pay more attention to the case.
vi) Unfortunately, we do not have a consensus about when pathologists must perform FISH evaluation. One group argues that all cases of DLBCL must be submitted for FISH for MYC, BCL2, and BCL6. Another group argues that only selected cases must be submitted for FISH for MYC, BCL2, and BCL6. For these, selected cases are: HGBL with atypical morphology for a DLBCL with expression for MYC and/or BCL2 in the immunohistochemistry.
vii) DHL or THL are HGBL with rearrangements of MYC and BCL2 and/or BCL6 determined by FISH examination. If the lymphoma is negative for these rearrangements, but has atypical morphological pattern with an atypical immunephenotype profile, including staining for MYC and/or BCL2, it is classified as HGBL, NOS.







