Abstract
Clinical manifestations of multiple myeloma are variable. The authors report a 68-year-old female who presented to the hospital with bilateral digital necrosis and dry gangrenous toes in both left and right feet. She was diagnosed with IgA-λ multiple myeloma associated with Type I cryoglobulinaemia. Emergency management consisted in hyperhydration (plasmapheresis was not available) and thromboprophylaxis. Necrotic digits were amputated. Chemotherapy (bortezomib, lenalidomide, and dexamethasone) was started with good initial evolution. This uncommon presentation can easily be missed, and clinicians should be aware of a possible underlying malignancy.
Key Points
1. This is a rare and atypical case of a patient with multiple myeloma associated with Type 1 globulinaemia, presenting with bilateral digital necrosis and dry gangrenous toes.2. The emergency management of the patient included hyperhydration and thromboprophylaxis; necrotic digits were amputated. The initiation of a course of chemotherapy (bortezomib, lenalidomide, and dexamethasone) demonstrated good initial results.
3. Clinical manifestations of multiple myeloma can vary widely. Rare manifestations of the disease may lead to misdiagnosis and delayed treatment.
INTRODUCTION
Multiple myeloma (MM) is a B cell malignancy characterised by abnormal proliferation of plasma cells that expand in the bone marrow and produce a monoclonal Ig, also known as M-protein.1 Several signs and symptoms of the condition are related to the excess amounts of the monoclonal Ig such as hyperviscosity syndrome, amyloidosis, renal failure, or autoimmune phenomenon.1 The monoclonal Ig can clump together and cause cryoglobulinaemia, usually of Type I. Cutaneous manifestations associated with Type I cryoglobulinaemia include Raynaud syndrome, acrocyanosis, livedo, urticaria, and cold-induced necrotic ulcers of the extremities.2 Only few reports of MM with digital necrosis have been described. The authors report the case of a 68-year-old female with MM revealed by gangrene of almost all fingers and toes.
CASE DESCRIPTION
A 68-year-old female, with medical history of neglected non-toxic multinodular goitre but no history of diabetes, venous thromboembolism, ischaemic heart disease, intravenous drug abuse, cigarette smoking, or alcohol dependence, presented to the hospital with a 3-week history of bilateral fingers and toes pain with blackish discoloration.
On admission, she was febrile (temperature: 38.7 °C), tachypnoeic (respiratory rate: 42 cycle/min), and tachycardic (pulse rate: 120 /min). Blood pressure and oxygen saturation were normal, as well as her Glasgow coma score (GCS). Her BMI was 23.4 (normal). Physical examination revealed bilateral digital necrosis and dry gangrenous toes (1st, 2nd, 3rd, and 4th) in both left and right feet (Figure 1) with normal peripheral pulses. Neck exam showed hard painless and nodular goitre. There were no palpable lymph nodes, hepatosplenomegaly, or bone pain. Cardiopulmonary auscultation showed lower-right lung dullness with crackling rales.
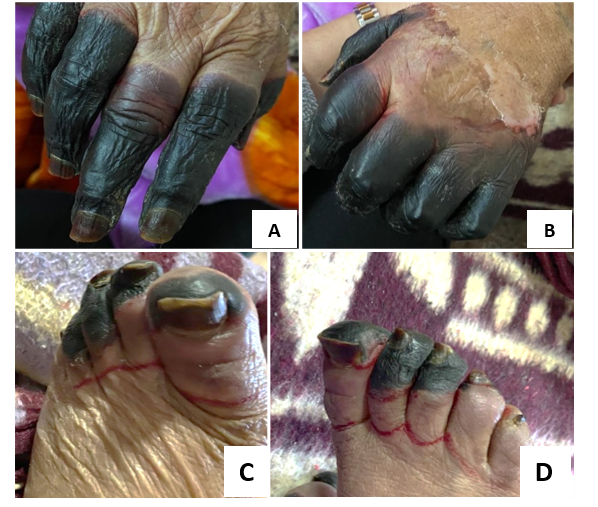
Figure 1: Gangrene in almost all of fingers and toes of the authors’ patient, seen on the right hand (A), left hand (B), left foot (C), and right foot (D).
The admitting laboratory results were as follows: haemoglobin: 9.9 g/dL; leukocyte count: 16 giga/L with 80% neutrophils; platelet count: 201 giga/L. Erythrocyte sedimentation rate: 90 mm/1st hour; total serum protein: 82 g/L (reference range: 62–87); albumin: 18.9 g/L (reference range: 35–50); calcium: 85 mg/L (reference range: 85–100); albumin-corrected calcium: 106.14 mg/L; C-reactive protein: 172.00 mg/L (reference range: 0.0–5.0); creatinine: 205.0 mmol/L (reference range: 53–115); fasting blood sugar: 4.2 mmol/L (reference range 3.9–6.1). Serum protein electrophoresis showed a monoclonal spike of 40.9 g/L in the β region and immunofixation electrophoresis confirmed that it involves IgAλ.
The serum-free κ was 19 mg/L, serum-free λ was 125 mg/L, and κ/λ ratio was 0.125 mg/L. Urine Bence Jones protein was positive. Cryoglobulin test was positive (qualitative research with cryoprecipitation method). Electrophoresis-immunofixation showed a monoclonal peak and typed the cryoprecipitate as IgA-L. β2 microglobulin was 6.2 mg/L (reference range: 0.97–2.64 mg/L), and lactate dehydrogenase 984 units/L (reference range: 120–300). Thyroid function was normal. Serological exams for HIV, hepatitis B virus, and hepatitis C virus were negative.
Under the suspicion of a connective tissue disease, a set of analyses were performed, including rheumatoid factor, anti-nuclear, anti-double-stranded DNA, anti-Sjögren’s-syndrome-related antigen A autoantibodies, anti-Sjögren syndrome antigen B, anti-nucleosome, anti-histone, anti-Smith antigen, anti-U3-ribonucleoprotein, anti-Jo-1, and anti-Scl-70 antibodies, which were all negative. Serum complement titrations were within normal values. Bone marrow aspirate revealed 30% infiltration by plasma cells including atypical forms, with no marked reduction in granulopoiesis or erythropoiesis (Figure 2). Conventional karyotype and fluorescent in situ hybridisation were normal.
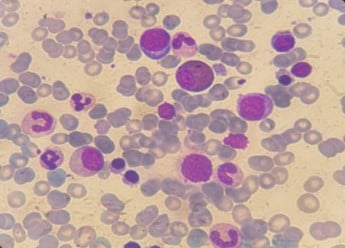
Figure 2: Bone marrow aspirate of our patient showing dystrophic plasma cells.
Chest X-ray showed lower-right lobar shadow. Whole body MRI showed no bone lesions. The diagnosis of MM with Type I cryoglobulinaemia was made. The patient was staged by the Revised International Staging System (R-ISS) as Injury Severity Score (ISS) III. Emergency management consisted in hyperhydration (plasmapheresis was not available), intravenous antibiotics, and thromboprophylaxis with low-molecular-weight heparin. All the necrotic digits were amputated (the decision was made in co-ordination with vascular surgery staff, the patient, and her family). The patient was started on bortezomib, lenalidomide, and dexamethasone. Initial evolution was good, with normalisation of fever and respiratory symptoms, and no further extension of the acrocyanosis. However, at Day 15 of the second chemotherapy session, she presented to the emergency room with 24 hours history of chest pain and dyspnoea. The diagnosis of massive pulmonary embolism was made, and the patient received curative anti-thrombotic treatment with low-molecular-weight heparin. Unfortunately, the patient died 48 hours later in the intensive care unit.
DISCUSSION
MM is a plasma cell dyscrasias afflicting mostly the elderly, over the age of 70.3 The International Myeloma Working Group (IMWG) consensus updated the diagnostic criteria for MM to include laboratory biomarkers, radiological, and histological findings in addition to hypercalcaemia, renal failure, anaemia, and bone lesions (CRAB) criteria.4 General symptoms such as fatigue, fever, night sweats, weight loss, and bone pain are classic manifestations of MM. Although, this malignancy exhibits a wide range of uncommon presentations, including ocular symptoms (proptosis, optic neuropathy, retinal haemorrhage, and detachment), neurological presentations (cranial nerve palsies, vertigo, and diabetes insipidus related to pituitary involvement), and gastrointestinal manifestations (acute and chronic pancreatitis, mesenteric ischaemia, and hepatosplenomegaly).5 In this case, MM was revealed by digital necrosis related to capillary lumen obstruction by Type I cryoglobulin precipitates. Cryoglobulins are abnormal plasma Igs that precipitate at low temperatures and dissolve upon rewarming. According to Brouet et al.,6 they are classified into three types:
1) Type I cryoglobulins are monoclonal (IgM > IgG > IgA), usually associated with B cell malignancies, most often MM, monoclonal gammopathy of undetermined significance (MGUS), or Waldenström macroglobulinaemia;
2) Type II cryoglobulins are polyclonal Igs associated with monoclonal Igs; and
3) Type III cryoglobulins involve only polyclonal Igs.
Type II and III cryoglobulins are usually IgM (κ > λ) and are referred as ‘mixed cryoglobulins’.7 They happen along with hepatitis C virus infection (70–90%), autoimmune diseases (Sjögren’s syndrome, followed by lupus and scleroderma), or B cell lymphoid malignancies. This patient’s cryoglobulinaemia was typed as IgA-L which is an extremely rare form. Rheumatoid factor activity is often found in mixed cryoglobulinaemia, unlike Type I cryoglobulinaemia, where it is rarely identified.8 Clinical manifestations associated with Type I cryoglobulinaemia are mainly cutaneous (purpura, Raynaud’s phenomenon, distal ulcers and necrosis, cold urticaria, and livedo). Rheumatologic, neurological, renal, gastrointestinal, and cardiopulmonary involvements are rare.9
According to a cohort study, 13.3% of patients with digital ischaemia associated with cancer had cryoglobulinaemia.9 The largest series of MM that presented as cryoglobulin-related symptoms was reported by Payet et al.10 Most of the patients had skin lesions (71 [4%]) among which only one patient presented with digital necrosis and gangrene. Treatment strategies for Type I cryoglobulinaemia with extensive necrosis involve plasmapheresis to quickly manage necrotising lesions and help their resolution. Specific chemotherapy prevents cryoglobulinaemia relapse and targets the underlying malignancy. In MM, bortezomib and lenalidomide are one of the most effective first line therapeutic agents.2,8,10
Unfortunately, despite an adequate initial management, MM associated with Type I cryoglobulinaemia has a poor prognosis. Most of the cases reported in the literature had fatal evolution (Table 1). In a retrospective study of 1,228 patients with MM, patients with skin involvement had significantly decreased overall survival compared to those without skin involvement (median: 28 versus 57 months).16
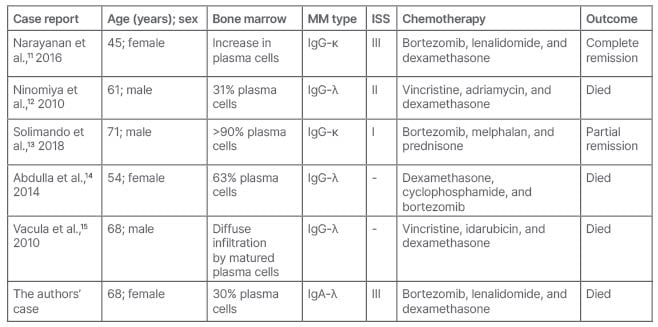
Table 1: Characteristics of patients presented with multiple myeloma presented as cryoglobulin Type I-related digital necrosis.
ISS: Injury Severity Score; MM: multiple myeloma.
CONCLUSION
Digital necrosis is a rare and atypical presentation of MM. Most often, it is related to Type I cryoglobulinaemia, a condition that requires specific management. It may lead to misdiagnosis and delayed treatment. Clinicians should be aware of this rare manifestation and consider MM diagnosis even in the absence of classical CRAB criteria.