Interview Summary
Warm autoimmune haemolytic anaemia (wAIHA) is a rare, life-threatening disorder caused by autoantibodies that lead to the premature destruction of erythrocytes (haemolysis). There is currently no licensed targeted therapy for wAIHA. Until recently, there has been little research attention on autoimmune haemolytic anaemias (AIHA), with few developments in the field over the past 20 years. The last 3 years have seen a surge in research interest in wAIHA, with the development of potential new therapies for this rare disorder. For this article, the EMJ conducted an interview in June 2024 with key opinion leader, Bruno Fattizzo, from the University of Milan, and Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy, to raise awareness of wAIHA, and explore recent advancements in research on this disease. Fattizzo, who has a wealth of experience and expertise in the clinical management of wAIHA, provided valuable insights into topics such as the clinical and quality of life (QoL) impact of wAIHA on the patient; current management of wAIHA, including steroids, rituximab, immunosuppressants, splenectomy, anticoagulant prophylaxis, and recombinant erythropoietin; and remaining unmet needs in the disease area. Also discussed were potential future therapies for this autoimmune disorder, including tyrosine kinase inhibitors, neonatal fragment crystallisable receptor (FcRn) inhibitors, and B cell activating factor receptor (BAFF-R) antagonists, and the challenges involved in managing patients with wAIHA. Finally, Fattizzo described the patient experience of living with wAIHA, proposed how best to raise awareness of wAIHA among healthcare professionals, the scientific community, patients, and the public, and outlined what the future of the management of patients with wAIHA might look like.INTRODUCTION
AIHA is an acquired, heterogeneous group of rare diseases with a prevalence of 17 per 100,000.1 This group includes wAIHA, cold agglutinin disease, mixed AIHA, paroxysmal cold haemoglobinuria, and atypical AIHA.2
wAIHA is a life-threatening AIHA typically caused by warm immunoglobulin G (IgG) autoantibodies,3-5 i.e., antibodies that react with antigens on the erythrocyte optimally at human body temperature (37 °C).4 It is the most common type of AIHA, comprising up to 80% of adult cases,4,6 and approximately 50% of paediatric cases.4
Fattizzo described wAIHA as an acute disease with an abrupt onset for many patients. At onset, anaemia is particularly severe. Patients usually need to be admitted to the emergency room, haematology ward, or internal medicine ward. Symptoms at onset and duringeach crisis of haemolysis include anaemia, jaundice, dark urine, fatigue, asthenia, and shortness of breath. wAIHA also has a psychological impact on the patient. These physical and psychological symptoms have a substantial impact on patient health and QoL.7,8
Until recently, there has been little research attention on AIHAs, with few developments in the field over the past 20 years. The last 3 years have seen a surge in research interest in wAIHA, with the development of potential new therapies for this rare disorder.
CLASSIFICATION OF WARM AUTOIMMUNE HAEMOLYTIC ANAEMIA AND OVERALL SURVIVAL
wAIHA can be classified as primary (approximately half of cases);4 drug-induced; or secondary to an underlying disease or disorder,3 such as lymphoproliferative neoplasms, autoimmune and infectious diseases, immunodeficiencies, solid tumours, or transplants.4 This disorder is associated with antibody-dependent cell-mediated cytotoxicity by macrophages and activated lymphocytes in the lymphoid organs and spleen, with the clinical severity of the disease determined by the ability of the bone marrow to compensate.4 wAIHA has considerable clinical heterogeneity and an unpredictable disease course, which might be explained by the complex interplay between the different pathogenic mechanisms of this disease.4 Secondary wAIHA is particularly complex because, in some patients, the associated condition is key to the development of wAIHA; whereas in others, wAIHA and the associated condition may arise from a shared genetic background.9 Therefore, treatment of the associated condition sometimes, but not always, improves the wAIHA.9 As a general strategy, treatment of the associated condition should be optimised, and the timing and intensity of wAIHA-directed treatment should be based on the individual patient.9
In a population-based study, presented at the 2024 European Haematology Association (EHA) Congress, the median overall survival (OS) for patients with wAIHA in Sweden was 8.5 years (95% CI: 7.0, unavailable).3 Specifically, the median OS for patients with secondary wAIHA was 6 years (95% CI: 4.3, unavailable) versus not reached at a median follow-up of 3.7 years for primary wAIHA (log-rank test p=0.003).3 Estimated 5-year OS for primary and secondary wAIHA was 77% and 58%, respectively.3
These results reinforce that wAIHA is a serious haematological disorder associated with a significant risk of mortality. Fattizzo noted that the OS reported in this study is low for a benign condition, and the higher mortality rate in patients with secondary wAIHA shows the importance of correct diagnosis and management of the associated condition. Fattizzo pointed out that patients with secondary wAIHA are often excluded from clinical trials, and clinicians may be apprehensive about administering wAIHA-directed treatments to these patients. In the context of limited clinical trial data, particularly on secondary wAIHA, Fattizzo considers that epidemiological studies with registry-based data provide useful insight into “the size of the problem”.
CURRENT MANAGEMENT OF WARM AUTOIMMUNE HAEMOLYTIC ANAEMIA
There is currently no licensed targeted therapy for wAIHA.9,10 In the past, due to the paucity of clinical trials on wAIHA, recommendations on diagnosis and therapy have often been based on expert opinion and some national guidelines.9 Several articles have been published recently on the topic.4,5,11,12 Consensus-based recommendations of the First International Consensus Group on diagnosis and therapeutic management of autoimmune haemolytic anaemias, including wAIHA, were published in 2020.9
Therapeutic interventions for wAIHA include steroids, rituximab, immunosuppressants, and splenectomy.4 Corticosteroids remain first-line therapy for wAIHA, although the addition of rituximab should be considered early in severe cases, and if there is no prompt response to steroids.9
Steroids
Over 85% of patients with wAIHA respond to initial treatment with steroids; however, more than two-thirds of these patients experience relapse.8 Furthermore, steroids are associated with substantial side effects, including hypertension, diabetes, osteoporosis, spontaneous fracture, and insomnia.13 Fattizzo highlighted that the relapsing nature of wAIHA following steroid treatment, and the side effects of these drugs, added to the burden of symptoms of the autoimmune disorder, have a marked impact on patient QoL.
Fattizzo explained that to limit the severity of relapse, patients should be followed up every month (including laboratory testing) until steroid dose is tapered (see recommendations below) and discontinued, and then every 3 months. At relapse, patients should start on a low dose of steroids to avoid the toxicity associated with high-dose steroids, then initiate rituximab as soon as possible.
The consensus-based recommendations for tapering of steroids are as follows. In patients responding to predniso(lo)ne at a dose ≥1 mg/kg, begin to taper after 14–21 days.9 Also begin to taper by 21 days in unresponsive patients.9 For patients responding well to predniso(lo)ne, consider stopping the treatment after at least 3 months after a complete response is achieved.9 Consider second-line treatment in unresponsive patients and those relapsing as the steroid is weaned.9
Rituximab
Fattizzo explained that rituximab is a monoclonal antibody drug ‘borrowed’ from the field of lymphomas and repurposed for use in wAIHA. The aim of treatment is to reduce autoantibody-producing B lymphocytes, thereby reducing the activation of haemolysis mediated by these autoantibodies. This strategy was successful and marked a ‘revolution’ in the treatment of wAIHA.
Over 75% of patients with wAIHA respond to rituximab; however, half of these patients relapse after 1 year.8 The remaining patients who are refractory to rituximab are treated with steroids, intravenous immunoglobulin, or splenectomy.8 Fattizzo explained that patients who initially respond to rituximab and experience a relapse at a later time can be treated again,4,14 provided that sufficient time has elapsed, to avoid severe or complete immunosuppression. These observations of refractoriness and relapse reflect a substantial unmet need in the treatment of wAIHA. In such cases, clinicians are encouraged to enrol eligible patients into clinical trials where possible.
Immunosuppressants
Immunosuppressants such as azathioprine, cyclophosphamide, methotrexate, cyclosporine, and mycophenolate mofetil are widely used in clinical practice for AIHAs.4 These treatments are usually administered alongside steroids and/or as steroid-sparing agents.4 The long-term side effects of these drugs have not been investigated in randomised controlled trials and are often underestimated.4
Splenectomy
Splenectomy is best deferred, if possible, but can lead to long-term remission in over two-thirds of patients.8 Splenectomy is associated with increased risk of thrombosis and infection.15,16
Anticoagulant Prophylaxis
Thrombotic manifestations are a major complication of wAIHA, with up to one in three adults with this disorder experiencing venous thromboembolic events.17 Anticoagulant prophylaxis is recommended for patients with wAIHA to reduce the risk of thrombosis.11
Recombinant Erythropoietin
The extent of bone marrow compensatory response is an important determinant of AIHA prognosis.12 One in three patients with AIHA has low reticulocyte levels and impaired bone marrow compensatory response to haemolysis.12 Recombinant erythropoietin is recommended in patients with AIHA, including wAIHA, to stimulate the bone marrow, thereby boosting bone marrow compensation, improving haemoglobin levels, and reducing the need for transfusion, particularly in the first 15 days of haemolytic crisis.12 In clinical practice, recombinant erythropoietin is more commonly used ‘on demand’ rather than as a continuous therapy.12
Therapeutic Algorithm
A therapeutic algorithm from the First International Consensus Group for wAIHA is shown in Figure 1.9
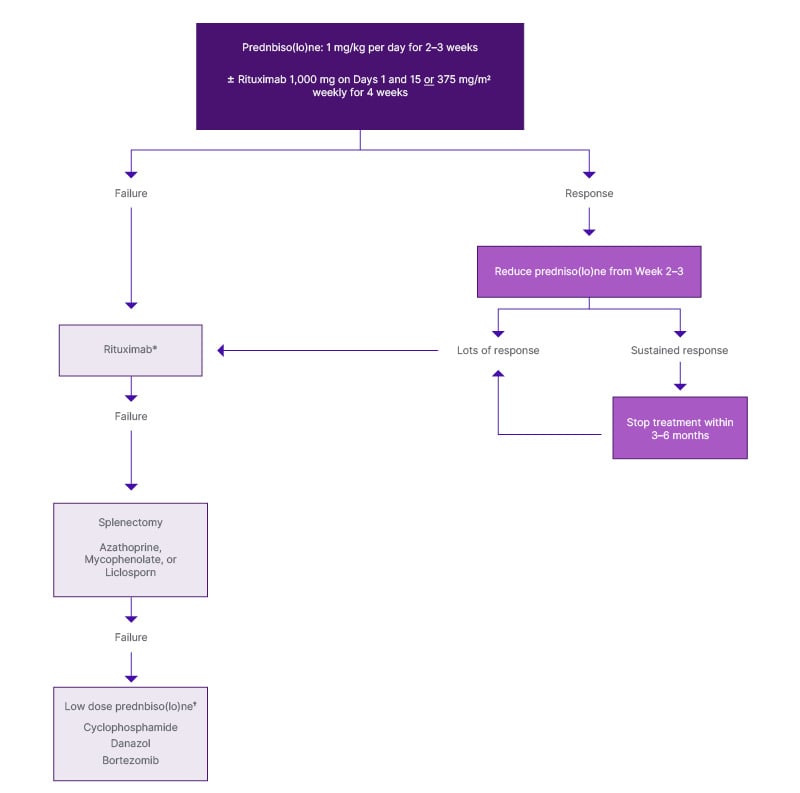
Figure 1: Therapeutic algorithm for warm autoimmune haemolytic anaemia.
Adapted from Jäger U et al. 2020.9
*If rituximab given first line, re-treatment may be considered if a sustained response was achieved. Otherwise, move to third line options.
†Predispose ≤10 mg ± a steroid-sparing agent
HSCT: haematopoietic stem cell transplantation.
POTENTIAL FUTURE THERAPIES FOR WARM AUTOIMMUNE HAEMOLYTIC ANAEMIA
There are several drug classes of potential clinical interest in wAIHA, including tyrosine kinase inhibitors, such as fostamatinib, sovleplenib (both spleen tyrosine kinase [Syk] inhibitors), and rilzabrutinib (a bruton tyrosine kinase [BTK] inhibitor); FcRn inhibitors, including nipocalimab; and BAFF-R antagonists, such as ianalumab and povetacicept (a dual APRIL/BAFF inhibitor). Also of potential interest are the CD19 B cell surface protein inhibitor, obexelimab, and the mTOR inhibitor, sirolimus.
There is limited experience with treatment of wAIHA in clinical trials. Several trials have been prematurely terminated because of a lack of efficacy compared with placebo. Fattizzo explained that clinicians and researchers in the field of wAIHA have been closely monitoring the improvements in the treatment of lymphomas, myelomas, and other B cell or plasma cell-driven diseases, with the aim to consider using drugs of particular interest in wAIHA.
Tyrosine Kinase Inhibitors
Although the Syk inhibitor fostamatinib has shown efficacy and is approved in immune thrombocytopenia (ITP),18 the results from a Phase III trial in patients with wAIHA show that this drug did not meet the primary endpoint compared with placebo.19 Fattizzo described these results as “not very encouraging”, and the further development of fostamatinib in wAIHA as uncertain. A Phase III open-label extension study of fostamatinib is ongoing and enrolling by invitation.20
Sovleplenib is a potent and highly selective Syk inhibitor that has shown significant improvement in efficacy in patients with primary ITP.21 This drug demonstrated a favourable safety profile and an encouraging haemoglobin benefit compared with placebo in patients with wAIHA in the Phase II part (21 patients) of a randomised, double-blind, placebo-controlled Phase II/III trial; the results of which were presented at EHA2024.21 Fattizzo suggested that these efficacy signals merit further investigation of sovleplenib, particularly as this is an orally administered drug, which is generally considered convenient for the patient. However, given that sovleplenib has a similar mechanism of action to fostamatinib, the likelihood of this drug reaching clinical practice for patients with wAIHA is unclear.
Neonatal Fragment Crystallisable Receptor Inhibitors
The FcRn functions as a recycling mechanism to prevent degradation and extend the half-life of IgG and albumin in circulation.22 Blocking FcRn is a non-immunotoxic strategy to remove pathogenic autoantibodies from circulation.23 Several FcRn inhibitors that selectively target IgG recycling are being investigated in autoimmune cytopenias, including efgartigimod in ITP,24 and nipocalimab in wAIHA.22 Fattizzo is interested to see the clinical picture develop with FcRn inhibitors, such as nipocalimab, in wAIHA, highlighting that these drugs may be an effective option because of the potential for a rapid lowering of IgG levels. Durable response with nipocalimab in wAIHA is being assessed during a 6-month treatment period in an ongoing placebo-controlled clinical trial.25
Ongoing Clinical Trials
There are ongoing active clinical trials with rilzabrutinib (Phase II),26 and sovleplenib (Phase II/III).27 There are also ongoing, actively recruiting clinical trials with obexelimab (Phase III),28 nipocalimab (Phase II/III),25 sirolimus (Phase II),29 ianalumab (Phase III),30 and povetacicept (Phase I/II).31 Fattizzo commented that these drugs may be potentially useful in the treatment of wAIHA, but this research is mostly at the early stage of clinical evaluation in this disease.
CHALLENGES IN THE MANAGEMENT OF PATIENTS WITH WARM AUTOIMMUNE HAEMOLYTIC ANAEMIA
According to Fattizzo, an important initial challenge in the management of patients with wAIHA is to exclude all other possible associated conditions, particularly lymphomas, lymphoproliferative disorders, and chronic lymphocytic leukaemia, because these may require different treatment. Furthermore, misdiagnosing other conditions, such as haemophagocytic lymphohistiocytosis (a complication of lymphoma) as wAIHA,32 and treating accordingly, can have serious consequences. Inborn errors of immunity (previously known as primary immunodeficiencies) are a further complication. Fattizzo explained that patients with autoimmunity may have a basic germline derangement of immunity that was insufficient to cause severe infections in childhood but may predispose them to develop autoimmunity in adulthood. Moreover, the receipt of long-term treatment with steroids and/or rituximab may further impair the immune system, and the patient may develop lymphomas. Another factor to consider before initiating steroids, rituximab, or other treatments is the presence of infection.
Further challenges outlined by Fattizzo in the clinical management of wAIHA include identifying the key pathogenic mechanisms in the patient at the specific haemolytic crisis, and understanding how to effectively sequence or combine current treatments and/or novel therapies that target these pathogenic mechanisms to provide efficacy and minimise toxicity.
Treatments that inhibit B cell production of autoantibodies, remove circulating autoantibodies, or boost the bone marrow compensatory response according to the key pathogenic mechanisms, at that time, in the patient, will provide a more individualised, precision medicine approach.
Identifying biomarkers of impaired bone marrow function, high activity of autoantibodies, and excessive activity of the B cell compartment is a further challenge to be addressed. These biomarkers will help clinicians to initiate treatment strategies that target the most important pathogenic mechanism in the patient with wAIHA.
PATIENT EXPERIENCE OF WARM AUTOIMMUNE HAEMOLYTIC ANAEMIA
Fattizzo explained that the data on patient experience of living with wAIHA were historically based on the physician’s impression of patient health and the availability of biomarkers. For example, studies with rituximab, the first ‘innovative’ treatment for wAIHA, did not include patient-reported outcomes (PRO). Nowadays, in the era of PRO measures in clinical trials, it is clear to see whether improvements in haemoglobin levels are accompanied by an improvement in QoL.
As reported by Love et al.7 at EHA2024, there is limited information describing the experience of the patient with wAIHA, and few publications to guide the development of a PRO strategy for clinical trials. Fatigue is a common symptom in patients with AIHAs, and the Functional Assessment of Chronic Illness Therapy Fatigue Scale (FACIT-Fatigue)/ Patient Reported Outcomes Measurement Information System (PROMIS) Fatigue 13a33 may be the most appropriate tool to assess fatigue as a clinical trial endpoint.7 However, Fattizzo highlighted the need for standardisation of QoL measures as there are no validated questionnaires for wAIHA.
RAISING AWARENESS OF WARM AUTOIMMUNE HAEMOLYTIC ANAEMIA
According to Fattizzo, education is key to raising awareness of wAIHA. In the last 5 years, there has been considerable effort made in the scientific and clinical community, such as conducting sessions and scientific workshops on classic haematology, including AIHA, at congresses. Pharmaceutical company-driven investigator meetings, and education initiatives for clinicians, have also helped to educate the clinical community. Fattizzo noted that further effort is needed to raise awareness of wAIHA during medical and healthcare training, and among specialists outside haematology, such as rheumatologists and emergency room (ER) healthcare professionals, to enable them to identify patients with this disease and establish appropriate short-term treatment.
Raising awareness among patients and the public is best achieved through the media, with digital media particularly relevant for young adults. Fattizzo suggested that patients should be informed that research in wAIHA is ongoing, their treatment is based on recommendations from the scientific community, and although this is a rare disease, they are not alone in living with this disorder. Strategies to communicate with patients about wAIHA vary between countries and institutions, with consultants and specialist nurses sharing the responsibility in some countries, such as the UK. Fattizzo noted that written patient information can be difficult for patients to interpret, and this type of communication may worry rather than reassure patients when they see terminology such as ‘mortality’, ‘crisis’, and ‘ER’. Patient advocacy groups and patient associations have a valuable role in digesting medical information about wAIHA and translating it into easily understandable language for the patient.
FUTURE MANAGEMENT OF WARM AUTOIMMUNE HAEMOLYTIC ANAEMIA AND CONCLUSIONS
Fattizzo emphasised the need to harness the treatment of patients with wAIHA based on the key pathogenic mechanism in each patient at the specific haemolytic crisis. Strategies include treatments that inhibit B cell production of autoantibodies, controlling the levels of circulating autoantibodies, and boosting the bone marrow compensatory response with erythropoietin. Tailoring
these treatments for each patient will enable a more individualised, precision medicine approach.
According to Fattizzo, a key goal for future clinical research is to establish whether there is a more effective drug than rituximab for the treatment of wAIHA, particularly in terms of relapse-free survival, and whether BTK inhibitors are a potentially suitable oral substitute for rituximab. A further aim is to understand whether modulating B cell activity with drugs such as ianalumab, povetacicept, or obexelimab, rather than B cell depletion with rituximab, is effective in the treatment of wAIHA.
The role of the FcRn inhibitor nipocalimab in the treatment of wAIHA is also of interest, in the context of the safety, tolerability, and efficacy, shown in patients with myasthenia gravis,34,35 rheumatoid arthritis,36 Sjögren’s disease,37 and haemolytic disease of the fetus and newborn.38 Fattizzo would like to see this drug available as first- or later-line treatment for patients with wAIHA. Finally, Fattizzo hopes to see the development of more potent and durable B cell inhibitors than currently available, and research to investigate whether T cells have a role in wAIHA.






