Abstract
Non-Hodgkin lymphoma (NHL) is the eighth most common malignancy worldwide. Diffuse large B cell lymphoma (DLBCL) is the most frequent subtype, accounting for >30% of NHL cases. Advances in novel approaches in the last two decades, such as immunotherapy with rituximab, have achieved improvements in terms of overall and long-term survival rates. The current standard of care for the first-line treatment of DLBCL is chemotherapy with rituximab plus cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisolone; this regimen achieves complete and sustained remission in approximately 60% of patients. Nevertheless, DLBCL relapses in 30–40% of patients, of which 10% develop refractory disease. Recent findings have demonstrated that substantial responses could be achieved after second or third-line treatments with combined chemotherapy. Since 2012, the aza-anthracenedione, pixantrone, has been approved as a single agent for relapsed or refractory DLBCL. The drug could be a new option as a bridging therapy to consolidate autologous or allogeneic stem cell transplantation, which in turn, can deliver prolonged durations of remission. Numerous clinical studies are ongoing that aim to improve salvage rates, outcomes, and access to stem cell transplantations for relapsed or refractory DLBCL. The development of novel targeted therapies or chemotherapeutics, such as pixantrone, will help to salvage more patients and achieve further sustained and complete responses without compromising their quality of life.
INTRODUCTION
Non-Hodgkin lymphoma (NHL) is the eighth most common malignancy worldwide,1 with diffuse large B cell lymphoma (DLBCL) being its most frequent subtype. DLBCL accounts for over 30% of cases of NHL.2 It is a rapidly growing and aggressive malignancy in which large B cells with high levels of mitotic activity spread into lymph nodes or other tissues outside the lymphatic system. DLBCL generally occurs in patients >50 years old, and is slightly more common in women.3
Over the last two decades, advances in novel therapeutic approaches, such as immunotherapy with rituximab, have achieved very good results in terms of overall and long-term survival.4-6 The current standard of care for the first-line treatment of DLBCL is chemotherapy with rituximab plus cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisolone, yielding complete and sustained remission in ~60% of cases.5
Despite these good results, between 30% and 40% of patients relapse following first-line therapy and an additional 10% present the refractory disease.6-8 As defined by the criteria of Cheson et al.,9 relapsed DLBCL is characterised by the appearance of any new lesion after a complete response (CR), while refractory DLBCL is defined as failure of <50% of lesions to reduce in size following initial treatment. In these clinical settings, the standard therapeutic option is to initiate high-dose therapy prior to either autologous or allogeneic stem cell transplantation (SCT). Patients who are ineligible for SCT or who fail after second-line therapy have a poor prognosis,10 but recent findings have revealed that they could benefit from alternative salvage therapies.11 Salvage therapies may also be used as a bridge to autologous or allogeneic SCT. The aim of this article is to provide an overview of advances and perspectives related to induction therapies as a bridge to transplantation in relapsed or refractory DLBCL (RR-DLBCL), as well as novel strategies in multiply relapsed DLBCL (MR-DLBCL).
MANAGEMENT OF RELAPSED OR REFRACTORY DIFFUSE LARGE B CELL LYMPHOMA
Management of Refractory Diffuse Large B Cell Lymphoma in Patients Eligible for Stem Cell Transplantation
Salvage chemotherapy as a bridge to autologous SCT is the standard therapeutic option for relapsed DLBCL and is successful in 30–40% of patients.12 High-risk, chemotherapy-sensitive patients with a low probability of success with autologous SCT may be oriented to allogeneic SCT. Rates of relapse and progression in high-risk patients are comparable for the two approaches, although allogeneic SCT is associated with higher rates of non-relapse mortality than autologous SCT.13,14
There are many salvage therapies available, mostly involving rituximab in combination with standard antineoplastic agents. The most frequently used combinations are as follows:15
- R-ICE: rituximab plus ifosfamide, carboplatin, and etoposide
- R-DHAP: rituximab plus cytosine, arabinoside, cisplatin, and dexamethasone
- R-GDP: rituximab plus gemcitabine, dexamethasone, and cisplatin
- R-ESHAP: rituximab plus etoposide, methylprednisolone, cytarabine, and cisplatin
- R-GemOx: rituximab plus gemcitabine and oxaliplatin
What is the Best Salvage Therapy?
The best chemotherapy regimens are those that provide the highest response rates with the most tolerable toxicity. There is still no clear evidence regarding the superiority of one regimen over the other. Two prospective randomised studies have compared available salvage therapies (CORAL [COllaborative trial in Relapsed Aggressive Lymphoma] and LY12) but have failed to detect any significant differences in clinical outcomes, as detailed below.
COllaborative trial in Relapsed Aggressive Lymphoma (CORAL) Study
In the multicentre Phase III CORAL study, 477 patients with CD20+ DLBCL during their first relapse or who had disease that was refractory to first-line therapy were randomly allocated (1:1) to three courses of R-ICE (243 patients) or three courses of R-DHAP (234 patients). In both groups, treatment was followed by high- dose chemotherapy with carmustine, etoposide, cytarabine, and melphalan (BEAM), and then autologous SCT.16,17 Response rates were 64% (95% confidence interval [CI]: 56–70%), and 63% (95% CI: 55–69%), respectively. Overall, 50% of patients were able to proceed to autologous SCT, mainly due to an insufficient response to the second-line therapy. There was no significant difference between the two rituximab (R-ICE and R-DHAP) regimens in terms of 3-year event-free survival (EFS) or overall survival (OS).
In the subpopulation that underwent autologous SCT, 122 patients received 1-year maintenance treatment with rituximab and 120 patients were assigned to observation only.16 At 4 years, no difference in EFS was observed between the rituximab maintenance group and the control group (52% versus 53%, respectively), although there was a 15% attributable risk of serious adverse events in the active therapy group. Rituximab is therefore not recommended as a maintenance therapy after autologous SCT.
In the subpopulation that failed to proceed to autologous SCT, 13 patients died and 6 withdrew consent.18 The remaining 203 patients were candidates for third-line chemotherapy, for which they had an overall response rate (ORR) of 39%, including 27% CR/unconfirmed complete response (CRu) and 12% partial response (PR) (Figure 1). Of these, 32% (n=64) of patients subsequently underwent autologous SCT (n=56) or allogeneic SCT (n=8). The authors concluded that, while third-line salvage chemotherapy for DLBCL can lead to a clinical response, with the chance for transplantation and long-term survival, the rates are still relatively low and there is an urgent need for new drugs.
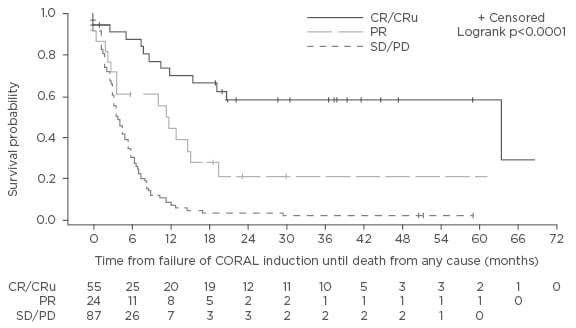
Figure 1: Overall survival (months) of 116 patients from time-to-treatment failure of CORAL induction according to the response to the third-line regimen.18
DLBCL: diffuse large B cell lymphoma; SD/PD: stable disease/progressive disease; PR: partial remission; CR: complete response; CRu: unconfirmed complete response; CORAL: COllaborative trial in Relapsed Aggressive Lymphoma study.
LY12 Study
The LY12 Phase III study was conducted by the National Cancer Institute of Canada in patients with RR-DLBCL. The aim was to compare two salvage therapies R-DHAP (n=310) and R-GDP (n=309),19,20 followed by autologous SCT, which was performed in 49% and 52% of cases, respectively. Four-year survival rates were comparable, with EFS rates of 26% and 26%, and OS of 39% and 39%, respectively. Notably, patients in the R-GDP treatment arm seemed to benefit from lower toxicity and higher scores for quality of life.
Other Studies
Other agents are being explored as bridging therapies in RR-DLBCL. As an example, in an open-label Phase II study, the combination of everolimus (an mTOR inhibitor) and rituximab were evaluated in 24 heavily pre-treated patients with RR-DLBCL.21 The ORR was 38% with three patients achieving CR and six patients with PR. Two of the patients with a CR were able to use this regimen as a bridging therapy and consequently underwent allogeneic SCT. After a follow-up period of 19 months, both patients were alive and disease-free.
Recently, the German High Grade Non-Hodgkin Lymphoma Study Group conducted a Phase II study in 84 patients with relapsed and refractory aggressive NHL to evaluate rituximab as an addition to graft-versus-host-disease prophylaxis following SCT.22 After myeloablative conditioning, all patients underwent allogeneic SCT, after which they were randomly allocated to receive rituximab or placebo (1:1). The results demonstrated that the addition of rituximab did not affect the incidence of graft-versus-host-disease or OS.
Other authors have also explored the addition of a new monoclonal antibody to a chemotherapy regimen. For example, a multicentre Phase II trial investigated the safety and efficacy of the anti-CD20 monoclonal antibody, ofatumumab, combined with ICE or DHAP as a second-line therapy in 61 patients with B cell lymphomas, including RR-DLBCL.23 The ORR was 61% for a CR of 37% (stem cell mobilisation was successful in 43 out of 45 patients). In the subsequent randomised comparison with rituximab, there was no difference between the two arms in terms of relative risk or progression-free survival (PFS).24
Can we Predict the Outcomes for Patients with Refractory Diffuse Large B Cell Lymphoma?
The poor outcomes obtained in patients with RR-DLBCL indicate that there remains a substantial unmet medical need. A recent multi-cohort study, SCHOLAR-1 (Retrospective NHL Research) in patients with refractory DLBCL reported homogeneous outcomes with response rates of between 20% and 30% and a median OS of approximately 6 months.25 Some prognostic factors have been identified for salvage therapy in RR-DLBCL. In the CORAL study, after BEAM and autologous SCT, 3-year EFS and OS were not significantly different but the outcomes were dependent on prior rituximab use, relapse within the first 12 months, and secondary age-adjusted International Prognostic Index (IPI) (Figure 1).16 Poorer outcomes in patients with early relapse after autologous SCT (<12 months) are consistent with those of the PARMA trial and other earlier studies.12,26,27
As previously stated, rituximab-naïve patients from the CORAL study had higher response rates and 3-year EFS than patients previously exposed to rituximab (response rates: 83% versus 51%, p<0.001; and 3-year EFS, 47% versus 21%, p<0.001, respectively).16 These results are in accordance with data from a retrospective study on R-ESHAP in patients with or without previous exposure to rituximab.28 As many patients develop disease that is refractory to rituximab, the available evidence suggests that its role in salvage therapy should be reconsidered. This challenge should also be overcome by the development of new chemotherapy combinations and novel agents.29 Finally, relapsed patients appear to have a higher OS than refractory patients.30 A low-risk age- adjusted IPI at relapse was also associated with higher PFS and OS rates.31
Management of Refractory Diffuse Large B Cell Lymphoma in Patients Not Eligible for High-Dose Therapy and Autologous Stem Cell Transplantations
A substantial proportion of patients are not eligible for high-dose chemotherapy followed by autologous SCT. This may result from advanced age or comorbidities, as they are refractory to second-line treatment, or because they express a wish not to undergo the treatment. Patients who are ineligible for high-dose chemotherapy followed by autologous SCT as described in the bone marrow transplant guidelines have distinctly lower survival rates.10,32,33 Treatment options comprise enrolment in Phase I or II clinical trials, palliative care with radiotherapy, rituximab therapy, and optimal supportive care.34
Management of Multiply Relapsed Diffuse Large B Cell Lymphoma
The standard of care for patients experiencing a second relapse is not clearly established and prognosis is extremely poor.35 Third-line chemotherapy may be attempted in chemosensitive patients, with the objective of achieving sufficient response to initiate allogeneic SCT. Allogeneic SCT appears to be the main option in MR-DLBCL, in the event that a histocompatible donor is available for a patient. This option offers two main advantages, namely the infusion of tumour-free stem cells and the graft-versus- lymphoma effect.36-38
In a retrospective study of the GITMO (Gruppo Italiano Trapianto di Midollo Osseo) database, 165 patients who underwent autologous SCT relapsed and were subsequently treated with allogeneic SCT.39 The results showed an ORR of 49%, with 43% of patients achieving CR and a further 5% obtaining PR. On the other hand, myeloablative conditioning with high-dose chemotherapy can generate higher transplant-related morbidity and non-relapse mortality. Thus, non-myeloablative or reduced-intensity conditioning approaches have been and continue to be evaluated.40-44
Chemotherapy can also be effective in MR-DLBCL. The CORAL study investigators conducted a retrospective analysis of patients failing second-line therapy (R-ICE or R-DHAP) and who could not proceed to autologous SCT (n=203).11 Third-line therapy included ICE (19%), DHAP (18%), gemcitabine-containing (14%), and miscellaneous regimens (32%), with or without rituximab. ORRs were lower than those of second-line therapies in the intent-to-treat analysis (39% versus 63%), but still acted as a bridging therapy in 32% of patients who underwent high-dose chemotherapy followed by autologous SCT (n=56), or high-dose chemotherapy followed by allogeneic SCT (n=8). In this third-line setting all patients were ineligible for (allogeneic) SCT, and a non-negligible proportion of this cohort benefited from autologous SCT.
In the subgroup of patients who were able to undergo a transplant, the median OS was 11.1 months, with a 1-year OS of 42%, compared with a median OS of 3.3 months (1-year OS, 16%) in patients who did not undergo transplantation (p<0.0001). OS was influenced by secondary age-adjusted IPI at the point of failure. However, the type of third-line regimen did not affect the outcomes, nor did the type of SCT (autologous or allogeneic). In a multivariate analysis, the IPI at relapse and SCT independently predicted OS (hazard ratio [HR]: 2.41 and 0.38, respectively) (Table 1).
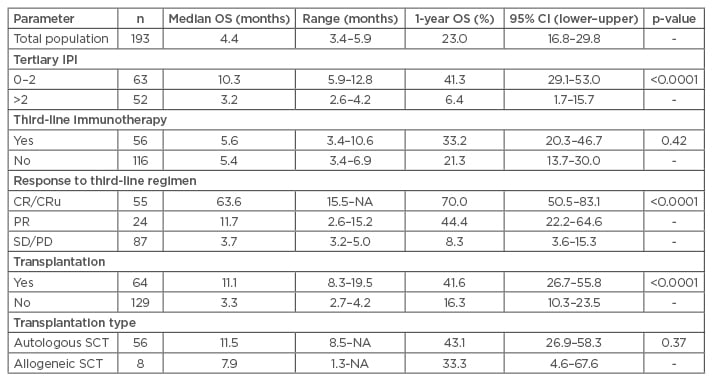
Table 1: Overall survival according to prognostic factors.18
SCT: stem cell transplantation; CI: confidence interval; CRu: complete response undetermined; IPI: International Prognosis Index; NA: not available; OS: overall survival; SD/PD: stable disease/progressive disease; PR: partial remission.
Overall, it appears that prolonged remission can still be achieved in an acceptable proportion of MR-DLBCL patients, with effective salvage regimens acting as bridging therapies to enable SCT. However, an improvement in salvage rates is a crucial requirement that needs to be addressed; the development of novel therapeutic agents will improve outcomes that allow more patients to be treated with SCT.
Pixantrone in Multiply Relapsed B Cell Non-Hodgkin syndrome
Pixantrone dimaleate is a novel aza-anthracenedione with unique structural and physiochemical properties,45,46 whose effects on DNA damage induction and cell death appear to be different from those of doxorubicin and anthracyclines. Pixantrone impairs mitotic fidelity, resulting in aberrant mitosis. The mechanism of action and efficacy of pixantrone seem to be independent of p53 status and influenced by checkpoint kinase 1 inhibition.47-49
The development of pixantrone was initiated to address severe cardiotoxicity issues related to the anthracyclines. As pixantrone lacks an iron-binding site, it has less potential to produce reactive oxygen species and does not form toxic drug-metal complexes. Pixantrone has also been demonstrated to be selective for Type II topoisomerase in stabilising enzyme-DNA complexes, which has also been hypothesised to explain the attenuated cardiotoxicity.50 These advantages, along with less alcohol metabolite formation in cardiac tissue, account for the limited cardiac toxicity potential. Thus with pixantrone, there are no dose restrictions or warnings related to prior anthracycline use. However, as stated in the summary of product characteristics, patients with prior cumulative doses of doxorubicin or equivalent exceeding 450 mg/m2 should receive careful risk versus benefit consideration before receiving treatment with pixantrone.45
Pixantrone (Pixuvri®, CTI BioPharma Corp.) has a conditional European marketing approval for monotherapy in adults with RR or MR aggressive B cell NHL. This authorisation was based on the results of the Phase III PIX301 study. This open- label, randomised, controlled, multicentre, single-agent, Phase III trial evaluated the efficacy of pixantrone in the treatment of patients with relapsed, aggressive NHL after more than two combination chemotherapy regimens.51,52 Pixantrone was therefore assessed in the setting of third-line therapy and beyond. A total of 140 patients were randomised (1:1) into two treatment arms: pixantrone (on Days 1, 8, and 15 of 28-day cycles) or a comparator (at the physician’s discretion) for up to six cycles of treatment. The primary endpoint was independently assessed CR and CRu. Approximately half of the patients had previously received rituximab therapy.
In the intention-to-treat population, the primary endpoint CR/CRu rate at the end of study was 24.3% (median duration 9.6 months) and 7.1% (median duration 4.0 months) for the pixantrone and the comparator arm, respectively (p=0.009).52 The ORR at the end of the study reached 37.1% versus 14.3% (p=0.001). Most importantly, pixantrone achieved CR/CRu in patients that had PR, stable disease, or progressive disease from prior intensive salvage therapies. Overall, 82% (14 out of 17) of the pixantrone CR/CRu had a suboptimal response to these prior therapies and yet went on to achieve a CR with pixantrone. Median PFS survival was longer in the pixantrone arm (5.3 months; 95% CI: 2.3–6.2) than in the comparator arm (2.6 months; 95% CI: 1.9–3.5; p=0.005; HR: 0.6 [95% CI: 0.42–0.86]). Similar results were observed in the subpopulation of patients with DLBCL (4.6 months; 95% CI: 2.3–6.5 versus 2.1 months; 95% CI: 1.8–3.2; p<0.001; HR: 0.47 [95% CI: 0.30–0.71]).
In a post hoc analysis of the trial, the population with a histologically confirmed diagnosis after central review was subdivided according to previous rituximab use and whether they received the study treatment as a third or fourth-line.53 In this population, when it was used in the third or fourth-line, pixantrone monotherapy was more effective than comparator in terms of response (CR: 23.1% versus 5.1%, p=0.047; ORR: 43.6% versus 12.8%, p=0.005; Table 2, Figure 2). These results were found to be consistent in patients who had previously received rituximab. Moreover, the observation of a 45.0% ORR with pixantrone in those patients versus 43.6% in the whole population (Table 2) suggests that treatment with pixantrone may have more potential as a bridge to transplant.
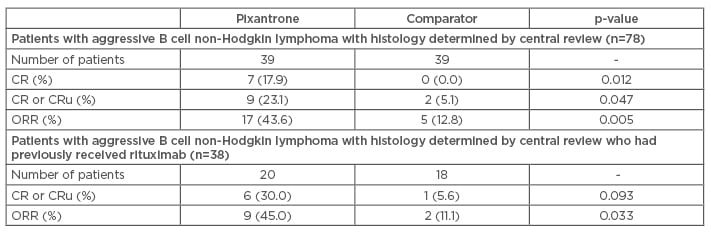
Table 2: Response rates until the end of study in patients with aggressive B cell non-Hodgkin lymphoma receiving their third or fourth-line therapy.53
CR: complete response; CRu: unconfirmed complete response; ORR: overall response rate.
p-value versus comparator (Fisher’s exact test).
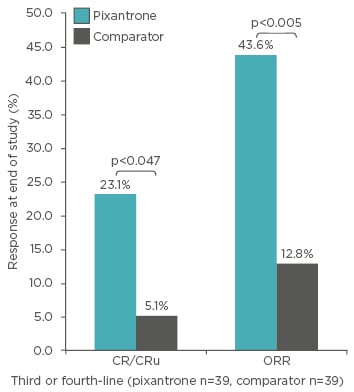
Figure 2: Post-hoc analysis: Response at end of study in patients with aggressive B cell non-Hodgkin lymphoma (determined by central review) receiving their third or fourth-line of therapy.53
CR: complete response; CRu: unconfirmed complete response; ORR: overall response rate.
p-value versus comparator (Fisher’s exact test).
The most common Grade 3 or 4 adverse events for pixantrone in the entire study were cytopenias (uncomplicated, non-cumulative neutropenias, leukopenias, or thrombocytopenias), with an incidence of febrile neutropenias in 7.4% of cases for pixantrone versus 3.0% for comparator agents.52 No high-grade treatment-emergent alopecia, mucosal inflammation, or opportunistic infections were reported for pixantrone and the incidence of severe infections was low.45
Following these promising results, a larger scale randomised, active-controlled, multicentre Phase III trial (PIX306 study) was initiated and is currently recruiting participants.54,55 This trial aims to enrol 260 patients with RR B cell NHL or follicular Grade 3 lymphoma who previously received at least one rituximab-containing multi-agent therapy regimen and are not eligible for SCT. In the study protocol, patients are randomised (1:1) to pixantrone and rituximab combination or gemcitabine plus rituximab for up to six cycles of treatment. The primary endpoint is PFS; secondary endpoints comprise OS, CR rate, ORR, and safety outcomes.
The National Institute for Health and Care Excellence (NICE) published a final guidance document on February 26th 2014, on pixantrone monotherapy in RR or MR B cell NHL.56 Key clinical evidence from PIX301 allowed NICE to appraise pixantrone under the single technology appraisal process, which concluded that the available results demonstrated that pixantrone can be a therapeutic option in RR or MR B cell NHL in the third or fourth-line settings. Cost-effectiveness assessments revealed that pixantrone was a cost-effective therapeutic option with an incremental cost-effectiveness ratio of £22,000 per quality-adjusted life year gained. Thus, pixantrone is currently licensed under the indications cited above; further clinical studies will evaluate its benefits in other therapeutic situations alone or in combination therapies.
IS PIXANTRONE A CANDIDATE FOR BRIDGING TO AUTOLOGOUS STEM CELL TRANSPLANTATION?
The trial evidence with pixantrone indicates that this agent can induce CR in patients with relapsed aggressive NHL.52,53 Clinical responses have even been observed in patients with disease that was refractory to standard salvage chemotherapy. The response appears to be sufficiently strong to hypothesise that mobilisation of stem cells may be an option. Although this has not been demonstrated in the clinical trial setting, this finding implies that bridging to autologous SCT may be feasible with pixantrone and should be explored further.
FUTURE PERSPECTIVES AND CONCLUSION
Patients failing after second-line therapy for DLBCL suffer from an overall poor prognosis. Nevertheless, recent findings have demonstrated that substantial responses could be achieved after second or third-line treatments with combined chemotherapy. The novel agent, aza-anthracenedione pixantrone as a third-line approach or beyond, has demonstrated efficacy in the same patient setting. Thus, these therapies might be successfully used as a bridge to consolidation of autologous or allogeneic SCT, which in turn can deliver prolonged remission durations. Numerous clinical studies are being conducted to improve salvage rates and outcomes of RR-DLBCL. The development of novel chemotherapeutic agents or targeted therapies will certainly help to salvage more patients and achieve further sustained CRs without compromising the quality of life of the patient.







