A key aim of modern cancer medicine is the improvement of treatment efficacy and overall patient health using targeted therapies. One of the most interesting approaches is the use of ‘targeted drug delivery vehicles’. The features most common to targeted drug delivery vehicles are a nanoparticle that encapsulates or binds the therapeutic agents to be delivered, and functional ‘targeting’ molecules attached to the nanoparticle’s surface that promote specific binding or uptake by the cancer cells (Figure 1). In this way, high local drug concentrations can be delivered to cancer cells, while limiting ‘off-target’ exposure to bystander cells (Figure 2).
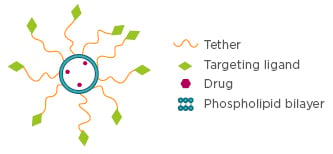
Figure 1: Liposomal drug delivery vehicle decorated with cancer-cell targeting ligands that are conjugated to the liposome through a long and flexible tether. Inside the liposomal lumen, the therapeutics to be delivered are encapsulated.
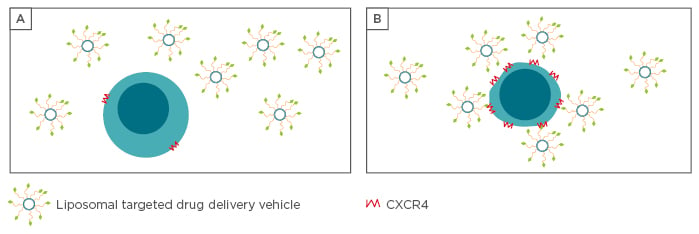
Figure 2: Illustration of the concept behind targeted drug delivery to cancer cells.
A) Healthy B-cell with low CXCR4 expression leads to most liposomes passing by without CXCR4 complexation, with the drugs remaining encapsulated. B) Cancerous B-cell with CXCR4 overexpression leads to a greater proportion of liposomal attachment.
The CXCR4 chemokine-receptor is an attractive target for drug delivery: it is overexpressed in >20 cancers. Furthermore, binding of its ligand CXCL12 may increase the survival of the neoplastic cells or promote their entry into protective cellular niches.1,2 Antagonism of the interaction between CXCR4 and CXCL12 has been shown to increase sensitivity to chemotherapeutic agents both in vitro and in vivo.2-4 Therefore, targeted drug delivery vehicles that both bind and antagonise CXCR4 could allow specific drug delivery to cancer cells, while simultaneously blocking CXCL12-induced chemoprotection. We have designed and synthesised a new compound (BAT1) that binds to CXCR4 and blocks the intracellular signals induced by its ligand (CXCL12). A key feature of BAT1 is its flexible amine-terminated tether that permits attachment to nanoparticle drug delivery vehicles, such as liposomes (through lipid conjugation). We hypothesised that employing BAT1 as a nanoparticle targeting agent will fulfil two simultaneous functions: the blockade of responses to CXCL12 and the specific delivery of therapeutic agents to CXCR4-expressing target cells.
In this study, chronic lymphocytic leukaemia (CLL) was chosen as the in vitro disease model; CLL is a good model for a targeted nanoparticle approach because the neoplastic cells are accessible within the blood, marrow, or lymphoid organs, and the expression and function of CXCR4 are central to the disease biology.5 Initial work focussed on the three-step synthesis of BAT1, confirming its structure and purity at each synthetic step. Using cultures of primary human CLL cells, BAT1 was then demonstrated to have high affinity for the CXCR4 receptor and to successfully disrupt the interaction between CXCR4 and CXCL12, halting associated signalling responses. When BAT1 was conjugated to a fluorescent cargo, specific dose-dependent targeting was observed.
Future work will focus on the encapsulation of relevant chemotherapeutics in liposomes conjugated to BAT1. Initially, this will employ model drugs such as doxorubicin, for which liposomal formulations are already in clinical use (e.g. Doxil), before moving towards CLL-specific therapeutics such as Bcl-2-targeting small interfering RNA. One challenge in the field of targeted drug delivery is the intracellular trafficking of the nanoparticle; many drug delivery vehicles will be taken up through endocytosis; therefore, the cargo is in danger of degradation in the endosomes. For this reason, later work will explore directed uptake using additional liposomal decoration that may direct uptake pathway. This will make use of the nanoparticles’ characteristic high surface area-to-volume ratio, which allows multiple functional molecules to be conjugated to nanoparticles’ surfaces.







