Multiple myeloma (MM) is a malignant neoplasm of plasma cells that accumulate in bone marrow, leading to bone destruction and marrow failure. MM accounts for approximately 1.8% of all haematologic and solid cancers; moreover, MM accounts for >15% of haematologic malignancies in the USA. MM is typically sensitive to different classes of cytotoxic drugs, both as front-line treatment and as treatment for relapsed disease. Unfortunately, even if responses are typically durable, nowadays MM is not considered curable with current approaches. However, treatment of MM has been rapidly evolving due to the introduction of new classes of drugs, such as proteasome inhibitors, immunomodulatory drugs, histone deacetylase inhibitors, and monoclonal antibodies, alongside new indications for old classes of drugs, such as alkylating agents. Furthermore, there is increasing understanding of MM tumour biology, creating the rationale for new combinations of drugs and the development of new therapies. Discovery of the associated cytogenetic abnormalities confirm the hypothesis that MM is a heterogeneous disease, suggesting that risk- adapted therapies and individualising treatment will further help to improve patient management.
With the aforementioned in mind, the aim of this study was to evaluate, in relapsed and refractory MM, the retrospective experience of several treatments, including old and novel agents, in order to compare real-world experience with the results of clinical trials.
During the EHA Congress 2018, four retrospective, observational studies were presented. They aimed to evaluate, in a real-life setting, a cohort of heavily pretreated patients affected by relapsing/refractory MM. Efficacy and safety data were evaluated (Table 1). Data on efficacy and safety of these real-life experiences seem to be highly comparable to those of major trials adopting the same regimen in the same clinical setting, demonstrating how these therapies can be considered feasible salvage therapeutic options, even in heavily pretreated patients.
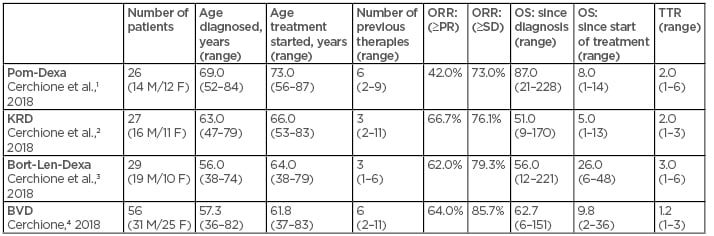
Table 1: Novel agents for management of relapsed and refractory multiple myeloma: Real-life experiences in AOU Federico II, Naples, Italy.
Bort-Len-Dexa: bortezomib-lenalidomide-dexamethasone; BVD: bendamustine-bortezomib-dexamethasone; F: females; KRD: carfilzomib-lenalidomide-dexamethasone; M: males; Pom-Dexa: pomalidomide-dexamethasone; PR: partial response rate; ORR: overall response rate; OS: overall survival; SD: standard deviation; TTR: time to response.
In particular,
- Pomalidomide-dexamethasone has shown significant efficacy and a very good compliance, thanks to oral administration, in a particularly severe group of heavily pretreated patients relapsed and refractory to all available therapeutic resources.1,5
- Carfilzomib-lenalidomide-dexamethasone has shown significant efficacy in a particularly severe group of patients relapsed and refractory to all available therapeutic resources and lenalidomide. In particular cases, it could be considered as a bridge to a second autologous or allogenic stem cell transplant.2,6
- Bortezomib-lenalidomide-dexamethasone triplet, thanks to a notable proven synergistic mechanism of action between bortezomib and lenalidomide, has shown significant efficacy in a severe group of heavily pretreated patients relapsed and refractory to bortezomib and lenalidomide.3,7
- The triplet bendamustine-bortezomib-dexamethasone has shown significant efficacy in a particularly severe group of patients relapsed and refractory to all available therapeutic resources. In particular cases, it could be considered as a bridge to a second autologous or allogenic stem cell transplant.4,8







