BACKGROUND AND AIMS
Dasatinib is a potent inhibitor of lymphocyte-specific protein tyrosine kinase (Lck), which plays a pivotal role in T-cell receptor (TCR) signalling, with immediate downstream targets including ZAP70 and linker of activated T cells (LAT), which are also implicated in regulatory T cell (Treg) development. Signal transducer and activator of transcription 5 (STAT5), the downstream target of IL-2, also plays a critical role in Treg differentiation and maintenance of FOXP3 expression through its binding of the promoter region of the FOXP3 gene. A subset of patients on dasatinib develop clonal large granular lymphocytosis (LGL) which is associated with immune-mediated toxicity and improved outcome.1,2 The authors hypothesised that a reduction in Treg frequency and function would correlate with the expansion of clonal LGL populations in patients receiving dasatinib.
METHODS
Phosphoflow cytometry was performed in Tregs and T effectors to assess the effect of dasatinib on TCR signalling.3 Cells were activated with the phosphatase inhibitor H2O2 and were analysed for phosphorylation of ZAP70, LAT, and STAT5. A gating strategy of CD4+/CD25+/FOXP3+/CD127lo cells was used for identification of Tregs, with FOXP3hi/CD45RA-ve cells denoting effector Tregs. Intracellular flow cytometry was also performed on T effectors after stimulation with OKT3, assessing the impact of dasatinib on cytokine expression, including TNFα, interferon-γ, IL-2, -4, and -10.4
RESULTS
Fifteen patients with chronic myeloid leukaemia (dasatinib [n=11], imatinib/nilotinib [n=4]) and five healthy controls were recruited. Patients on dasatinib had lower Tregs compared with the non-dasatinib group (mean CD3+ cells: 1.3% versus 2.0%; mean CD4+ cells: 2.6% versus 4.3%; p=0.01 and p=0.007, respectively). Dasatinib-treated patients also had a lower percentage of effector Tregs: 11.5% versus 22.1% (p=0.0097) (Figure 1A).
Patients on dasatinib had significantly reduced phosphorylation of ZAP70, LAT, and STAT5 compared with the non-dasatinib group, in CD4+ cells, CD8+ cells, and Tregs, following stimulation (phosphorylated STAT5 mean increase in median fluorescence intensity [MFI] of 4.1 versus 21.7 in CD4+ cells; 6.1 versus 28.2 in CD8+ cells; and 4.4 versus 22.6 in Treg [p=0.0001, p=0.0001, and p=0.001, respectively]) (Figure 1B).
Mean absolute increase in IL-2 expression was also lower in patients on dasatinib (0.9 versus 7.0 in CD4+ cells; 0.3 versus 3.0 in CD8+ cells; p=0.001 and p=0.014, respectively). Five patients on dasatinib had reversal of CD4:CD8 ratio and lymphocytosis, in line with clonal LGL populations, with TCR clonality confirmed by PCR. These patients had lower Tregs compared with other patients on dasatinib, with a mean CD3+ cells percentage of 0.9% versus 1.8% (p=0.035). A lower increase in MFI within isolated Tregs following stimulation was seen in this group, when compared with patients on dasatinib with normal CD4:CD8 (phosphorylated ZAP70: 1.8 versus 0.8, p=0.024; phosphorylated LAT: 4.4 versus 1.4, p=0.05; phosphorylated STAT5: 7.4 versus 2.0, p=0.15) (Figure 1C).
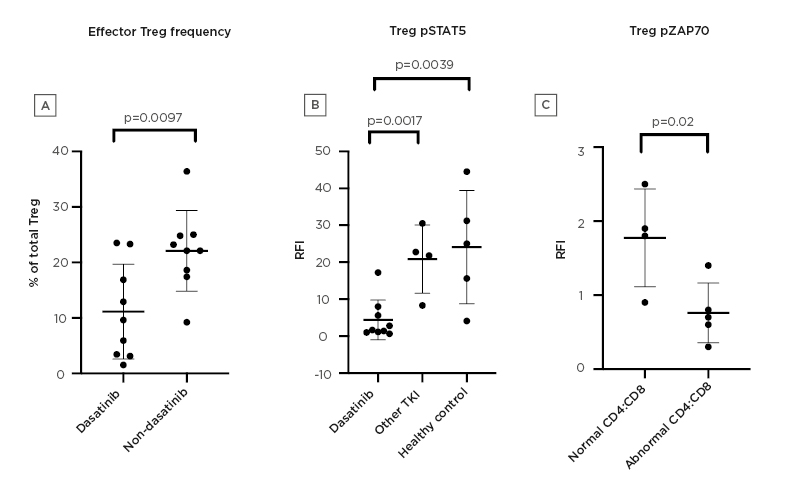
Figure 1: Effects of dasatinib on regulatory T cells.
A) Proportion of effector regulatory T cells (Tregs) in the dasatinib group compared with the non-dasatinib group. B) RFI of pSTAT5 in Tregs in the dasatinib group, other TKI group, and healthy controls. C) RFI of ZAP70 in Tregs in dasatinib patients with CD8+ lymphocytosis compared with the dasatinib group with normal CD4:CD8.
pSTAT5: phosphorylated signal transducer and activator of transcription 5; RFI: relative fluorescence intensity; TKI: tyrosine kinase inhibitor; Treg: regulatory T cell.
CONCLUSION
Dasatinib potently inhibits signalling from the TCR in T effectors but also in Tregs, as indicated by inhibition of phosphorylation of ZAP70 and LAT, as well as STAT5, which is essential for transcription of FOXP3. Dasatinib-treated patients have a reduction in proinflammatory cytokine expression within T effectors, with the most significant inhibitory effect seen against IL-2. Tregs have abundant expression of the IL-2 receptor on the cell surface and binding leads to STAT5 signalling.
A subset of patients on dasatinib with clonal LGL populations have further reduction in frequency and function of Tregs as assessed by signalling from the TCR and IL-2 receptor. These findings may explain the mechanism of lymphocytosis in this group and could be used to predict improved outcomes with dasatinib.







