BACKGROUND AND AIMS
Patients with haematological malignancies (HM) may experience significantly compromised quality of life (QoL) as a result of the disease and its treatments.1-4 It is paramount to seek and capture patients’ unmet needs in routine clinical practice.5,6 Until recently, there was no specific patient-reported outcome (PRO) measure for patients with HM to identify symptoms, the impact of the disease and of treatment on patients’ QoL in routine haematological practice.7-8 A new tool, HM-PRO, was developed by the the European Hematology Association Scientific Working Group for ‘Quality of Life and Symptoms’ (EHA SWG QoL & Symptoms) to measure PRO in individuals with HM in clinical practice.9-10
The HM-PRO consists of two scales: Part A (24 items) measuring the ‘impact on patients’ QoL’; and Part B (18 items) measuring ‘signs and symptoms’ (S&S) experienced by the patients. Part A had 4 domains: physical behaviour ([PB] 7 items), social wellbeing (3 items), emotional behaviour (11 items), and eating and drinking habits (3 items). Higher scores represented higher impact.
The aim of the study was to determine the value of the HM-PRO in daily clinical practice in Russia and to test its application for evaluating a patient’s condition during different stages and states of the disease.
METHODS
Patients aged ≥18 years old with different HM completed the Russian version of the HM-PRO before seeing the haematologist in out- or inpatient settings. Haematologists then reviewed patients’ responses during the consultation, as well as completed clinical and demographic information. They also reported on the feasibility of the HM-PRO in clinical practice. Descriptive statistics and parametric and nonparametric tests at a significance level of p<0.05 were applied.
A total of 192 patients and 29 haematologists from five tertiary hospitals in Moscow and St. Petersburg, Russia, were included in a cross-sectional study. Among patients, 93 (48%) were male and the mean age was 50.7 (standard deviation [SD]: ±14.8) years. Distribution according to the diagnoses was as follows: acute myeloid leukaemia (n=43), aggressive non-Hodgkin lymphoma (n=21), chronic lymphocytic leukaemia (n=10), chronic myeloid leukaemia (n=47), Hodgkin lymphoma (n=21), indolent non-Hodgkin lymphoma (n=7), myelodysplastic syndromes (n=14), multiple myeloma (n=22), myeloproliferative neoplasms (n=4), and others (n=3). Out of 192 patients, 58 (30%) patients had stable disease, 120 (63%) were in remission, and 14 (7%) had progressive disease. There were 79 (42%) inpatients and 113 (58%) outpatients. As for physicians, 10 (34.5%) were male, mean age was 36.1 (SD: ±11.4) years, and mean duration of employment was 10.9 (SD: ±10.1) years.
RESULTS
Physicians reported that the HM-PRO was useful for patient management in 155 cases (81%), which included all stages: at diagnosis, during treatment, and at follow-up. In Part A, items from domains PB and emotional behaviour were considered the most useful by haematologists in 38% and 34% of the patients, respectively, in particular: ‘difficulty with physical activity/sports’ (40%), ‘difficulty with work’ (28%), ‘worrying about treatment’ (21%), and ‘worrying about future health’ (18%). In Part B, the most informative signs and symptoms were ‘problems with energy level’ (37%), ‘tiredness’ (32%), ‘hair loss’ (20%), and ‘night sweats’ (18%). Physicians reported that the HM-PRO was useful for all patients with progressive disease and for the majority in remission or with stable disease. PB and S&S scores were significantly worse in patients with progressive disease (Table 1).
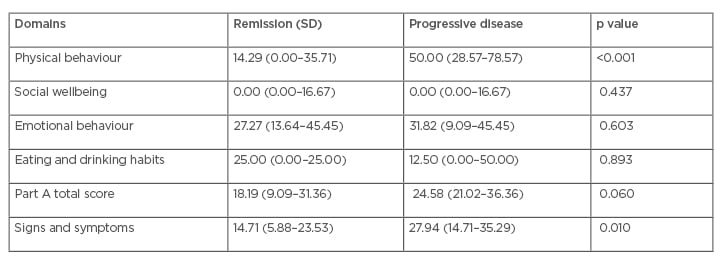
Table 1: Haematological malignancies patient-reported outcome medians and interquartile ranges.
SD: stable disease.
Impacts on QoL (Part A total score) and S&S (Part B total score) were significantly worse in patients with progressive disease compared to those in remission or with stable disease (p<0.01). Patients with progressive disease experienced moderate effects on QoL and S&S whereas those in remission or with stable disease reported small effects on QoL and S&S. Impacts on QoL and S&S were significantly worse for inpatients compared to outpatients (p=0.003). QoL impact was significantly worse in patients whose PRO data affected medical decisions compared to those that did not affect medical decisions (p<0.001).
CONCLUSION
The authors demonstrated that the newly developed PRO measure for HM is an informative tool to identify the impact of the disease and its treatment on patients’ QoL as well as S&S in this patient population in routine haematological practice. From the haematologists’ perspective, the HM-PRO is a valuable tool to capture patients’ needs regardless of the stage and state of the disease. The use of HM-PRO in clinical practice may facilitate the discussion between patients with HM and clinicians on an individual basis to better deliver patient-centred care.







