Search of more effective treatment for patients with acute myeloid leukaemia (AML) who are ineligible for intensive chemotherapy remains challenging. Monotherapy with hypomethylating agents (HMT) constitute the backbone therapy in this patient population, associated with a modest clinical benefit.1,2 Recently, the combination of HMT with agents against determinate novel targets has resulted in unprecedented response rates with a low toxicity profile. In this context, FLT3 inhibitors, targeting one of the most recurrently mutated genes in AML, have demonstrated significant activity in different clinical settings, such as frontline chemotherapy combined with intensive chemotherapy (midostaurin),3 as monotherapy for salvage therapy (quizartinib, gilteritinib),4-6 or as maintenance to prevent relapse after allogeneic hematopoietic cell transplantation (sorafenib).7
Gilteritinib is a type I FLT3 inhibitor with capability to bind both the monomeric (ITD mutation) and dimeric (D835 TKD mutation) forms of the FLT3 receptor. Pre-clinical work suggests a possible synergistic antileukaemic effect of gilteritinib when combined with azacytidine (AZA),8 and supports the clinical evaluation of this combination. This was the basis of a Phase I/II clinical trial aimed to explore the added benefit of combination of gilteritinib plus AZA in newly diagnosed FLT3mut+ AML ineligible for intensive induction chemotherapy.9 This trial consisted of an initial phase with a safety cohort, which evaluated feasibility and was aimed to define the recommended dose of gilteritinib in combination to AZA for a randomised phase, currently on-going. In this abstract, the results of a safety cohort of 15 patients are presented. Patients enrolled in this safety cohort received escalating doses of oral gilteritinib (80 or 120 mg/day) on Days 1–28 in combination with AZA (75 mg/m2/day) on Days 1–7. Treatment was continued in 28-day cycles until lack of a clinical benefit or unacceptable toxicity. Safety and tolerability were the primary endpoints of the safety cohort; antileukaemic activity was also assessed.
Fifteen patients (median age: 76 [range: 65–86]) were enrolled into the safety cohort (80 mg gilteritinib, n=9; 120 mg gilteritinib, n=6). All except one patient harbored a FLT3 mutation (ITD alone, n=10; TKD alone, n=3; ITD and TKD, n=1; none, n=1). Despite a high frequency of adverse events (AE) observed, frequent (>25% of patients) grade ≥3 AE corresponded to common events in this patient population, such as febrile neutropenia and cytopenias. Eight patients experienced fatal AE, none of which were related to treatment; three patients died in an early treatment phase due to septic shock (Day 2), respiratory failure (Day 6), and cerebral haemorrhage (Day 17). No patient presented a severe increase of liver enzymes (i.e., aminotransferase/alanine aminotransferase >3 X Upper Limit of Normal [ULN] and/or total bilirubin >2 X ULN) or significant QTcF interval prolongation, >500 msec. Only one dose limiting toxicity (DLT) consisting of an early episode of tumour lysis syndrome was observed in a patient who received 80 mg gilteritinib plus AZA, with no further episodes at the same or higher gilteritinib dosing. Given this unique DLT episode from the 11 DLT evaluable patients, the decision to proceed to the randomised portion (AZA plus gilteritinib versus AZA) at a dose of 120 mg gilteritinib for the combination treatment arm was adopted.
In this cohort of patients, 10 out of 15 patients (67%) achieved as best response as composite complete remission (CR; with incomplete haematological recovery: CRi), including four bona fide CR and six CRi. Two additional patients achieved a partial response (PR), giving an overall response rate (CR+CRi+PR) of 80%. Six of these CR/CRi patients maintained their response at last follow-up, between 220 and 377 days after treatment start (Figure 1). More than half (n=8/15; 56%) of the patients had a treatment duration lasting more than 6 months, while nine patients discontinued treatment (death, n=4; relapse; AE; physician decision; sponsor decision; subject withdrawal, n=1 each) and six patients remained on treatment.
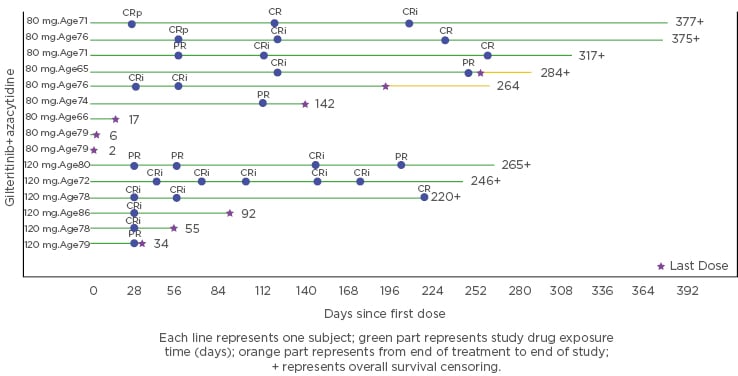
Figure 1: Graph depicting response obtained and treatment duration of the combination of azacytidine and gilteritinib in the trial safety cohort.
CR: complete remission; CRi: complete response with incomplete haematological recovery; CRp: complete response without complete platelet recovery; PR: partial response.
In conclusion, gilteritinib was safely combined with AZA without unexpected toxicity in this population of elderly, unfit AML patients, and 120 mg gilteritinib was the chosen dose for the randomised Phase II of the trial. Moreover, this combination therapy induced a significant proportion of antileukaemic responses in newly diagnosed FLT3mut+ AML patients, suggesting an added benefit and warranting further exploration.







