For high-risk haematological patients, in particular those with acute leukaemia, there is limited information on the use of peripherally inserted central catheters (PICCs) as a primary vascular access device. In other settings, the use of PICCs as an alternative to other central venous catheter (CVC) devices, particularly for prolonged infusions of cytotoxic agents, blood products, and/or other supportive therapy, is becoming very frequent in cancer patients.1,2
The Hematology Department of University Federico II, Napoli, Italy is conducting a Phase IV randomised trial3 (ongoing), comparing PICCs versus traditional CVCs as a front-line venous access device in patients affected by myeloid or lymphoblastic acute leukaemia, undergoing intensive chemotherapy for remission induction.
The primary endpoints of the trial are the most important complications of central venous access, such as the occurrence of catheter-related bloodstream infections and/or thrombosis. Secondary endpoints are the occurrence of other catheter-related complications, such as pneumothorax or catheter occlusion, and patients’ quality of life (QoL), evaluated through a questionnaire assessing daily activities.
From April 2015 to February 2017, 152 consecutive patients were enrolled and randomly assigned (1:1) to PICC (Arm A) or traditional CVC (Arm B) (Table 1). The inclusion criteria for the trial were age >18 years, expected survival >4 weeks, and in need of central venous access (long-term >4 weeks). Exclusion criteria were ongoing uncontrolled systemic infection, presence of significant thrombosis/ stenosis in arm or central veins, and inability to communicate and/or to sign informed consent. All insertions were followed by chest X-ray and intra-cavitary electrocardiogram for tip location.
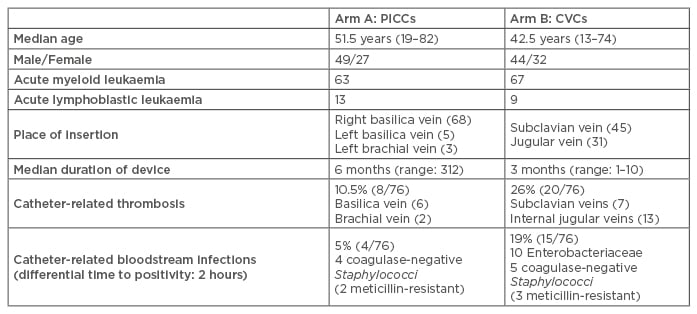
Table 1: Trial details.
CVCs: central venous catheters; PICCs: peripherally inserted central catheters.
In Arm A, 76 open-ended, power-injectable PICCs, in new generation polyurethane, were inserted in 76 patients, while in Arm B, untunneled heparin-coated Vialon CVC (Becton-Dickinson, Franklin Lakes, New Jersey, USA) were inserted by the Seldinger technique in 76 patients.
Overall, the median duration of in situ catheter placement was 6 months (range: 3–12) in Arm A versus 3 months (range: 1–10) in Arm B. In Arm A, catheter-related thrombosis occurred in 8 patients and catheter-related bloodstream infections in 4 patients. In Arm B, 20 cases of catheter-related thrombosis and 15 cases of catheter-related bloodstream infections were observed. Thus, PICCs were significantly associated with fewer major complications than traditional CVCs (catheter-related thrombosis: 10.5% in Arm A versus 26% in Arm B, p=0.01 by chi-squared test; catheter-related bloodstream infections: 5% in Arm A versus 19% in Arm B, p=0.007 by chi-squared test) (Figure 1).
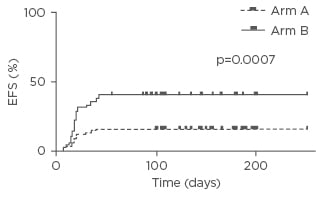
Figure 1: Event-free survival.
EFS: event-free survival.
The preliminary observations of this Phase IV randomised trial suggest that the use of PICCs represents an important advance in terms of decreasing the complication rate and improving QoL for high-risk haematological patients affected by acute leukaemia. In particular, the authors have shown a statistically different improvement in the PICC (Arm A) median duration of device, catheter-related thrombosis, bloodstream infections, procedure-related complications, and QoL.







