BACKGROUND AND AIMS
The authors have previously reported their Phase I clinical trial regarding a combination treatment of CD19- and CD22-specific chimeric antigen receptor (CAR)-T cells for post-transplant relapsed B-cell acute lymphoblastic leukaemia (B-ALL). In the trial, which included both paediatric and adult patients, the first CD19 CAR-T therapy resulted in a complete remission (CR) rate of 85% (23 out of 27 cases); furthermore, 21 patients received subsequent CD22 CAR-T cells (six cases withheld from the second cell therapy) and the overall survival (OS) and event-free survival (EFS) rates at 18 months were 88.5% and 67.5%, respectively.
To investigate the long-term efficacy of combination treatment with CD19 and CD22 CAR-T cells in these patients with relapsed B-ALL after allogeneic hematopoietic cell transplantation (allo-HCT), the 5-year survival outcome was followed up.
MATERIALS AND METHODS
Except for the 27 patients previously reported, an additional three cases relapsed only with bone marrow minimal residual disease (MRD), who were not eligible for the trial but were administered CD19 and CD22 CAR-T cells following the same protocol in the same time period, were included in this follow-up study. CAR-T cells were transfected by lentiviral vectors encoding second-generation CARs composed of CD3ζ and 4-1BB, the interval time between two cell infusions was 1.5–6.5 months. The first cell infusion was performed between December 2017–October 2019, and the cut-off date for the last follow-up was as 31st December 2023.
RESULTS
Three MRD relapsed cases turned MRD negative after first CD19 CAR-T. Among 24 patients who finished both CD19 and CD22 CAR-T therapy, with a median follow-up of 64.4 (95% CI: 50.6–68.6) months, 12 patients relapsed and the late relapse (more than 2 years after first CD19 CAR-T cell infusion) occurred in three cases (25%). Of relapsed patients, six died of disease progression and six were still alive after treatment with other therapies (four received second allo-HCT). More encouragingly, the other 12 patients remained in sustained remission since first CD19 CAR-T (n=11) or second CD22 CAR-T (n=1, who was with extramedullary disease and achieved partial remission after CD19 CAR-T). Kaplan–Meier survival analysis showed that the 3-year OS and EFS rates were 79% (95% CI: 57–91) and 54% (95% CI: 33–71), respectively, while the 5-year OS and EFS rates were 75% (95% CI: 53–88)and 50% (95% CI: 29–68), respectively (Figure 1).
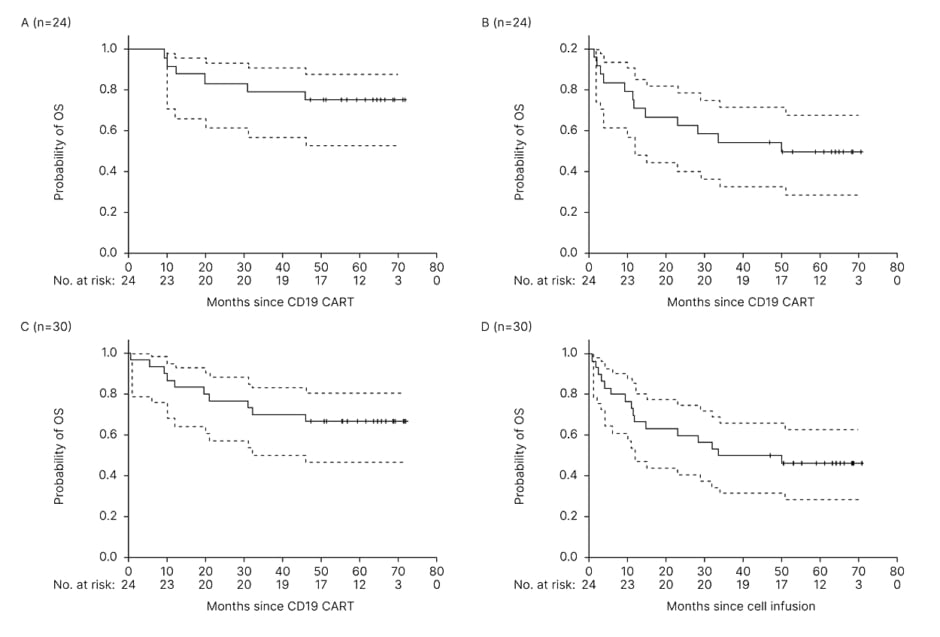
Figure 1: The probabilities of overall survival and event-free survival, analysed by the Kaplan–Meier method.
OS (A) and EFS (B) in 24 patients who received both CD19 and CD22 CAR-T cells. OS (C) and EFS (D) in all 30 intention-to-treat patients including six cases without undertaking the second CD22 CAR-T.
CAR: chimeric antigen receptor; EFS: event-free survival; OS: overall survival.
In all 30 intention-to-treat patients, including six cases without receiving second CD22 CAR-T cells, the OS and EFS rates were 70% (95% CI: 50–83)and 50% (95% CI: 31–66) at 3 years, and 67%(95% CI: 47–80) and 46% (95% CI: 28–63) at 5 years, respectively.
After each cell infusion, CR/partial remission patients with incomplete blood cell count on Day 30 had cell count recovery in 1.3–6.7 months; 13 severe infections (Grade 3 or greater) beyond 1 month and within 2 years were observed in 11 patients, presenting with 11 lung infections (Grade 3, n=10; Grade 4, n=1) and two skin varicella-zoster virus infections (Grade 3). Most infections occurred within 1 year (76.9%; 10/13).
CONCLUSION
In patients with relapsed B-ALL post-HCT, who previously had an extremely poor prognosis, the authors’ combination treatment of CD19 and CD22 CAR-T cells tremendously improved long-term survival with a 5-year OS of 75% and EFS of 50%.






