BACKGROUND AND AIMS
Acute myeloid leukaemia (AML) usually presents in older patients over the age of 65 years.1 A large proportion of such patients are frail or have comorbidities that make them poor candidates for intensive therapies.2 Venetoclax-based lower-intensity regimens are now established as standard therapy for older or unfit patients with AML.3,4 However, initial clinical trials of venetoclax did not incorporate objective methods for delineating patients who are considered ‘unfit’ for intensive chemotherapy. Consequently, the benefit of venetoclax-based regimens in truly unfit patients has been debated, and experts have advocated for randomised controlled trials against intensive chemotherapy.5
The authors conducted a retrospective study to address these questions.6
MATERIALS AND METHODS
The authors compared outcomes of 85 patients with newly diagnosed AML, treated on a prospective Phase II trial of 10-day decitabine and venetoclax (DEC10-VEN), with a historical cohort of patients treated with intensive chemotherapy containing cytarabine of at least 1 g/m2/day.7 The historical cohort of 85 patients was selected from over 1,300 patients using propensity score matching. To better understand outcomes in patients deemed unfit by objective methods, the authors used a previously validated treatment-related mortality scoring that classified patients at low versus high risk of 30-day mortality from intensive chemotherapy.8
RESULTS
The two cohorts of patients treated with DEC10-VEN and intensive chemotherapy were well balanced in terms of baseline characteristics. More than 50% of patients were >70 years, >30% patients had Eastern Cooperative Oncology Group (ECOG) performance status scores of 2 or higher, nearly 30% patients were at high risk of early mortality form intensive chemotherapy, and nearly two-thirds of patients had European LeukemiaNet (ELN) adverse risk disease. The authors found that DEC10-VEN led to significantly higher rates of complete remission compared to intensive chemotherapy (62% versus 42%; p=0.01), lower rates of relapse (34% versus 56%; p=0.01), lower 30-day mortality (1% versus 24%; p<0.01), and longer overall survival (OS) at 12.4 months versus 5.0 months (Figure 1). Patients at both low risk as well as high risk of early mortality from intensive chemotherapy derived benefit with DEC10-VEN and had significantly longer OS.6 Subgroup analysis for OS showed benefit with DEC10-VEN over intensive therapy in most subgroups and multivariable analysis confirmed improved outcomes with DEC10-VEN over intensive therapy for most outcomes including complete remission rates, early mortality, and OS.
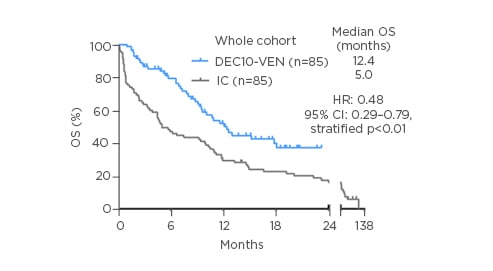
Figure 1: Overall survival in patients with newly diagnosed acute myeloid leukaemia treated with DEC10-VEN, and a propensity score-matched cohort of patients treated with intensive chemotherapy.
CI: confidence interval; DEC10-VEN: 10-day decitabine with venetoclax; HR: hazard ratio; IC: intensive chemotherapy; OS: overall survival.
CONCLUSION
The authors concluded that in this retrospective analysis, DEC10-VEN showed better outcomes compared to intensive chemotherapy in newly diagnosed AML. Patients who were at both low risk as well as high risk of early mortality with intensive chemotherapy appeared to benefit from DEC10-VEN. These results have implications for the design of future clinical trials incorporating venetoclax-based regimens. The authors commented that clinical trials evaluating the role of venetoclax with hypomethylating agents in younger and older ‘fit’ patients are currently ongoing at their institution.







