Presenters: Rena Yadlapati,1 Jolien Schol,2,3 Jan Tack,2,3 Nawalf Bent-Ennakhil,4 Maura Corsetti,5 Enrique de-Madaria,6 Trond Engjom,7 Gerard Clarke8
1. Esophageal Diseases and Motility, University of California San Diego Center for Esophageal Diseases, USA
2. Department of Gastroenterology, University Hospitals Leuven, Belgium
3. KU Leuven, TARGID, Belgium
4. Takeda Pharmaceuticals International AG, Glattpark-Opfikon, Switzerland
5. Translational Medical Sciences, Nottingham Digestive Diseases Biomedical Research Centre, UK
6. Gastroenterology Department, Alicante University General Hospital, Alicante, Spain
7. Haukeland University Hospital Medical Clinic, Section for Gastroenterology, Bergen, Norway
8. Department of Psychiatry and Neurobehavioural Science, and APC Microbiome Ireland, University College Cork, Ireland
Disclosure: Yadlapati has received consulting fees from Medtronic, Ironwood Pharmaceuticals, and Phathom Pharmaceuticals; funding to participate in an advisory board for RJS Mediagnostix; and has received research funding from Ironwood Pharmaceuticals. Schol has declared no conflicts of interest. Tack has given scientific advice to Adare, AlfaWassermann, Allergan, Arena, Bayer, Christian Hansen, Clasado, Danone, Devintec, Falk, Grunenthal, Ironwood, Janssen, Kiowa Kirin, Menarini, Mylan, Neurogastrx, Neutec, Novartis, and Noventure. Bent-Ennakhil is an employee of Takeda Pharmaceuticals International AG. Corsetti has provided clinical consultation and lecturing to Arena, RB, Mayoly, and Sanofi; and has received research funding from Sanofi. de-Madaria has received grants and/or consultation fees from Abbott, Mylan, and Takeda. Engjom has received grants and/or lecture fees from the Norwegian Gastroenterology Association. Clarke has received honoraria from Janssen, Probi, and Ipsen; research funding from Pharmavite and Fronterra; and consultancy fees from Yakult and Zentiva.
Acknowledgements: Writing assistance was provided by Nicola Humphry, Nottingham, UK.
Support: The publication of this article was funded by Abbott. Its Global Medical Affairs department selected the abstracts for inclusion.
Citation: EMJ Gastroenterol. 2021;10[Suppl 2]:4-14.
Meeting Summary
The United European Gastroenterology (UEG) Week Virtual 2021 illuminated a wide range of new insights and recommendations for gastroenterological disease. Referring to guidelines published earlier this year, Rena Yadlapati discussed the use of proton pump inhibitors (PPIs) for refractory gastro-oesophageal reflux disease (GORD), and Jan Tack highlighted the use of PPIs and probiotics for new therapeutic targets in functional dyspepsia. Jolien Schol and Nawal Bent-Ennakhil presented surveys that revealed a highly varied approach to functional dyspepsia in the real-world and highlighted the importance of personalised care and shared decision making in inflammatory bowel disease (IBD), respectively. A new algorithm for the treatment of constipation was described by Maura Corsetti, covering key causative factors to rule out, and the distinction between functional constipation and irritable bowel syndrome (IBS) with constipation (IBS-C). Enrique de-Madaria described a systematic review and meta-analysis that assessed the prevalence of pancreatic exocrine insufficiency (PEI) in patients with advanced pancreatic cancer, and the benefits of pancreatic enzyme replacement therapy (PERT) in this population, and Trond Engjom followed up with the results of a real-world study into the adherence to European guidelines for the use of PERT in chronic pancreatitis. Finally, Gerard Clarke discussed how the gut microbiome fits into the view of IBS as a disorder of gut–brain axis interaction.Proton Pump Inhibitors and Side Effects of Acid Suppression in Refractory Gastro-Oesophageal Reflux Disease
Rena Yadlapati
Yadlapati presented insights from the 2021 European Society of Neurogastroenterology and Motility (ESNM) and American Neurogastroenterology and Motility Society (ANMS) Consensus Report on refractory GORD.1 Yadlapati stressed that while it is important to begin by treating refractory GORD with lifestyle adjustments such as weight loss and sleep hygiene, the first-line pharmacotherapy is gastric-acid suppression with PPIs.
Patients with GORD can present with typical oesophageal symptoms such as heartburn or regurgitation,2 or with extra-oesophageal syndromes such as cough or laryngitis.3 Yadlapati explained that the efficacy of PPI therapy for symptom relief depends partly on the presenting symptoms, and also on whether patients have GORD proven by objective testing. PPI therapy appears to be reasonably effective in patients with proven GORD (relieving symptoms in 25–50%), but less so in patients with unproven GORD, particularly those presenting with atypical symptoms. Therefore, it is appropriate to trial PPIs in patients with unproven GORD and typical reflux symptoms, or those with proven GORD, but not in patients with isolated extra-oesophageal symptoms and unproven GORD.
When prescribing PPIs, Yadlapati emphasised several factors that should be considered. First, PPIs are acid-labile molecules and the presence of food in the stomach reduces their absorption. Therefore, PPIs should be taken 30–60 minutes prior to a meal. Second, there is a degree of pharmacologic variability between PPIs.4 In Yadlapati’s experience, switching PPIs can be effective if patients are not deriving sufficient benefit from their current PPI, and moving from a once-daily to a twice-daily dosing regimen can improve intra-gastric pH control. The metabolism pathways can also vary between PPIs, impacting their potential for interaction with concurrent medication.5 Third, Yadlapati underlined the importance of considering the 3–5-day lead time to achieve peak PPI concentration, and the occurrence of nocturnal acid breakthrough in some patients, when prescribing a PPI. In summary, clinically practical strategies to optimise PPI therapy include ensuring compliance and before-meal dosing; considering a higher dose or twice-daily dosing; and, if switching PPIs, considering intra-gastric acid suppression, potential drug-drug interactions, and using a more potent non-PPI agent if available.
If symptoms improve, clinicians and patients may consider stopping PPI treatment. Although some patients tolerate an abrupt cessation of PPI treatment, there is a theoretical risk of rebound gastric acid secretion, and some of Yadlapati’s patients have reported an increase in symptoms. Therefore, Yadlapati recommends a gradual taper over 1 month. If symptoms recur during tapering, then this is an indication for long-term therapy with the lowest effective PPI; however, objective GORD testing should be conducted before initiating lifelong therapy.
Yadlapati highlighted that some studies have identified an association between long-term PPI use and the development of adverse conditions such as dementia, kidney disease, and osteoporosis. However, Yadlapati emphasised that these studies are not definitive, and do not establish a causative relationship. Other studies, including meta-analyses, have shown that PPIs do not significantly increase the risk for adverse conditions other than intestinal infections.6 For minor side effects such as headaches, nausea, and diarrhoea, switching PPIs can be considered.
In Yadlapati’s practice, histamine (H2) receptor antagonists are commonly used as an adjunct to PPI therapy in patients with refractory GORD. Yadlapati uses PPIs as the first line of therapy because they are superior to H2 receptor antagonists in terms of their gastric acid suppression. Yadlapati explained that it is reasonable to trial night-time H2 receptor antagonists in patients who experience heartburn or chest-pain like symptoms at night, which could be acid mediated. However, Yadlapati pointed out that clinicians should bear in mind that about 50% of patients may also be experiencing tachyphylaxis. Yadlapati might also use H2 receptor antagonists in patients who are strongly averse to PPI therapy, and has found that, in conjunction with lifestyle management, mild cases of GORD can be managed in this way, illustrating that GORD treatment really needs to be personalised to each patient.
Management of Functional Dyspepsia in Europe: Expert Opinion in a Case-Based Delphi Approach
Jolien Schol
European evidence-based guidelines for functional dyspepsia recommend that diagnostic examination includes upper gastrointestinal (GI) endoscopy and Helicobacter pylori testing, and PPI therapy and H. pylori eradication are the only treatment options recommended by consensus.7
Schol introduced a study that evaluated the management approaches for functional dyspepsia used in the real-world. Using a Delphi approach, 15 case descriptions were evaluated by 33 experts across 16 European countries. In line with recommendations, all experts reported that they would test for H. pylori in patients with suspected functional dyspepsia, and they would treat positive cases to eradicate the infection.
For uncomplicated cases of functional dyspepsia that present with post-prandial distress syndrome (PDS), 67% of experts would order an upper GI endoscopy as a primary approach and 73% would order a duodenal biopsy. Abdominal ultrasound and coeliac serology were advocated by 55% and 42% of experts, respectively. A similar approach was used in patients who presented with epigastric pain syndrome (EPS) or with overlapping PDS/EPS. However, gastric emptying tests were more commonly used as a tertiary approach for the management of PDS (58%) than for EPS (15%). Experts were generally more likely to order gastric emptying tests overall in patients that presented with concomitant nausea or vomiting.
The presence of risk factors changed the management approach used by experts. In patients aged >60 years, all experts would order an upper GI endoscopy regardless of presentation, and more than half of experts (52–63%) would order a CT scan if initial therapy was not effective. Initial coeliac serology and CT scans were often performed in patients with concomitant weight loss (63–67% and 30–34% of experts, respectively), and gastric emptying tests in patients with persistent nausea or vomiting (88% of experts each).
In terms of treatment choices, experts used diet, PPI, or prokinetic therapy as a primary approach (27%, 36%, and 30% of experts, respectively) in patients presenting with PDS. For non-responders, the most prevalent treatments were prokinetics (42%) and neuromodulators (21%). In cases of EPS, the preferred first-line therapy primary was PPI (82%), followed by a neuromodulator in non-responders (36–67%). In cases with overlapping EPS/PDS, the predominant first-line treatment was a PPI (66%), with prokinetics (44%) commonly used in non-responders, and neuromodulators (66%) as the tertiary approach.
Treatment approaches to PDS were similar in older patients (>60 years), although a selective serotonin re-uptake inhibitor was more likely to be used in unresponsive patients, rather than a tricyclic agent. Patients with weight loss who were unresponsive to treatment were more likely to be treated with mirtazapine than with buspirone or tricyclic agents. In patients presenting with EPS, older age and weight loss had little effect on treatment choices. A prokinetic agent was commonly used in patients with concomitant nausea or vomiting (41% of experts), and an antiemetic was often introduced as a secondary approach in patients with vomiting (34% of experts).
In this study, domperidone was the most prescribed pharmacological treatment in uncomplicated cases of PDS, and Schol explained that while this drug is not recommended by European guidelines, it is used in Belgium because of the lack of available prokinetic alternatives. Schol stressed that potential side effects are always taken into account, and patients are screened using an ECG to detect QT prolongation prior to initiating therapy.
In contrast to evidence-based guidelines, expert opinion reveals a highly varied approach to functional dyspepsia, tailored to the presenting symptom, the presence of risk factors, and comorbidities. Treatment choices were highly variable, including different types of prokinetics and neuromodulators. These observations illustrate the richness of functional dyspepsia management in clinical practice.
New Drugs in Functional Dyspepsia
Jan Tack
The 2021 UEG and ESNM consensus on functional dyspepsia recommended that a patient who presents with chronic early satiation, postprandial fullness, epigastric pain, or burning should have an endoscopy to diagnose functional dyspepsia, and an H. pylori test.7 However, Tack stressed that in primary care, suspected functional dyspepsia can be treated empirically, with endoscopy reserved for patients over 40 or 50 years of age. H. pylori should be eradicated if patients test positive for the infection, after which a PPI at a standard dose for 4–8 weeks forms the first line therapy. Dietary adjustment and nutritional support are also recommended. If there is no benefit observed after PPI therapy, patients with EPS can be treated with low-dose tricyclic agents. The use of prokinetics, mirtazapine, or serotonin 1A receptor agonists for patients with PDS did not achieve consensus for recommendation, and the use of hypnotherapy or cognitive behavioural therapy for any form of functional dyspepsia also failed to achieve consensus.7
Although there is currently insufficient high-quality evidence to determine whether prokinetics are beneficial in functional dyspepsia,7,8 Tack highlighted a recent analysis suggests that some prokinetics may still prove to be useful in this field; Carbone F et al.,9 showed that itopride is associated with improvements across many Leuven Postprandial Distress Scale (LPDS) sub-scores.
Tack explained that the place of PPIs in the treatment of functional dyspepsia has changed, and their benefits are no longer considered limited to improving symptoms of pain and burning.
In the last decade, there has been an increase of research into the pathophysiology of functional dyspepsia, with the recognition that this condition is associated with increased numbers of mast cells and eosinophils in the duodenum, and a loss of mucosal barrier function.10,11 This is viewed as a new therapeutic target, and PPIs have been shown to improve both the Patient Assessment of Upper Gastrointestinal Symptom Severity Index (PAGI-SYM) and the LPDS along with a reduced elevation of duodenal eosinophils and improved duodenal barrier function in patients with functional dyspepsia.12 Tack feels that both eosinophils and mast cells are likely to work together in the duodenal mucosa, and that chronic inflammation may involve a vicious cycle of reciprocal activation. In this case, therapies that target either cell type could be effective in functional dyspepsia.
In Tack’s practice, roughly 50% of patients with functional dyspepsia have eosinophilic duodenitis and stressed that prevalence was similar in other countries. Although it is unlikely that eosinophil elevation has potential as a diagnostic marker, Tack believes it may represent a therapeutic target; however, Tack stressed that it is eosinophilic activation rather than the number of cells that is relevant in functional dyspepsia.
Another new concept in the treatment of functional dyspepsia is the use of probiotics. Tack described an 8-week controlled trial of combined Bacillus coagulans MY01 and Bacillus subtilis MY02 that showed significantly better responder rates (decrease in PDS score) in patients treated with these probiotics compared with the placebo.13 Responses were associated with a reduction in both IL-17A levels and the proportion of IL-17-positive T-helper cells, indicating that the probiotics had an anti-inflammatory effect.
One recent study indicates that eosinophils and mast cells may be targets for both PPIs and probiotics in functional dyspepsia. Lirentelimab is an antibody that targets the siglec-8 ligand, found selectively on the surface of these two cell types. A Phase II controlled trial, in patients with symptomatic eosinophilic gastritis and/or eosinophilic duodenitis (N=65), showed that lirentelimab significantly reduced GI eosinophil levels, induced a treatment response, and reduced symptom severity compared with placebo. This was a rapid effect that was durable over the 14 weeks of the trial and was also sustained over a 52-week open-label extension, with upper GI symptoms showing the most improvement (Dellon ES et al.,14 2020).
Like many biologic therapies, lirentelimab is likely to be an expensive drug, but Tack explained that it may be possible to target the siglec-8 ligand in other ways, with small molecules for example, or drugs could be used to inactivate eosinophils through alternate pathways. Tack also highlighted recent studies that suggest that the beneficial effects of dietary interventions may also be explained by the effect of the diet on eosinophil and mast cell activation and impaired mucosal permeability, including improvement in mucosal resistance in patients with IBS following a fermentable oligo-, di-, monosaccharides, and polyols diet.15,16
Finally, Tack posited that since lirentelimab affects both eosinophils and mast cells, and it is not clear which of these is most responsible for the anti-inflammatory benefits. It is possible that drugs that target mast cells, such as H1-, H2-, or leukotriene-receptor antagonists, might also be effective in functional dyspepsia.
Results from a Large Survey Exploring Patient Preferences for Treatment: Attributes in Inflammatory Bowel Disease Across Seven Countries in Europe
Nawal Bent-Ennakhil
Understanding patient preferences through shared decision making optimises treatment acceptance and adherence. Bent-Ennakhil reported the results from a survey aimed to explore patients’ preferences for treatment attributes of the currently available advanced therapies for IBD, including route of administration and expected treatment outcomes with respect to quality of life.
The cross-sectional online survey was conducted from 21st October 2020–31st January 2021 and included patients aged ≥18 years from seven European countries, who self-reported having, and had been treated for Crohn’s disease (CD) or ulcerative colitis (UC). Using discrete choice experiment questions, patients were asked to select hypothetical treatments for CD or UC, and the relative importance of treatment attributes was assessed. Patients were also asked about their quality of life and treatment preferences.
Of the patients who completed the survey, 360 had CD and 326 had UC. The mean age was 48 and 50 years, 71.9% and 57.7% were female, and the mean disease duration was 13.6 and 11.0 years for patients with CD and UC, respectively. The proportion of patients currently receiving treatment for CD and UC was 76.7% and 78.5%, respectively. Patients considered the most common reason for treatment switch to be failure to control IBD (CD: 41%; UC: 32%). The aspects of daily life most anticipated to improve with treatment were general well-being (CD: 75%; UC: 76%) and energy status (CD: 73%; UC: 69%).
For patients with UC, the most important attributes for treatment choice were route of administration and frequency of serious adverse events (AEs), and patients preferred a treatment that minimised the risk of serious or mild AEs. Both oral administration and subcutaneous injections were preferred to intravenous injections. Less important attributes of treatment choice were long-term remission, the ability of treatment to heal the bowel lining, 1-year corticosteroid-free remission, and the occurrence of mild AEs.
For patients with CD, the most important attribute for treatment choice was the risk of serious AEs that required hospitalisation. One-year remission and long-term remission and were also considered important treatment attributes. Bent-Ennakhil concluded that this study illustrated the variability between patients with CD or UC, in terms of their preferences for treatment attributes, highlighting the importance of personalised care and shared decision-making.
Pharmacological Treatment of Constipation
Maura Corsetti
There are two main categories of functional bowel disorder associated with chronic constipation: functional constipation and IBS-C.17 Patients with functional constipation present with straining, hard stools, a sensation of incomplete evacuation or anorectal blockage, the need for manual evacuation, or less than three spontaneous bowel movements per week.18 However, Corsetti explained that when these symptoms are also accompanied by abdominal pain then IBS-C should be suspected, particularly if the pain is relieved by defaecation or is worse when the patient is more constipated. Patients with IBS-C may also present with painful conditions outside of the GI tract, such as fibromyalgia, or chronic back pain, or pelvic pain, and interstitial cystitis.18 Corsetti emphasised that it is important to recognise IBS-C because it can be difficult to treat the constipation without exacerbating abdominal pain in these patients.19
If a functional defaecation disorder is suspected, additional tests are required to confirm diagnosis, such as an abnormal expulsion test, anorectal manometry, or imaging to detect impaired rectal evacuation.17,20 However, Corsetti stressed that it is important to be aware of the weaknesses of these investigations. For example, balloon expulsion tests may not be available in all medical centres; anorectal manometry is not able to distinguish between healthy subjects and those with functional constipation;21 and anorectal alterations revealed by defaecography have yet to be clearly identified as causative factors in functional constipation.22,23 Corsetti also emphasised that it is important to remember that opioid therapy can also trigger the development of functional defaecation disorders.24 Along with international colleagues, Corsetti reviewed the existing literature and published an algorithm to guide physicians in the treatment of patients with constipation (Figure 1).17
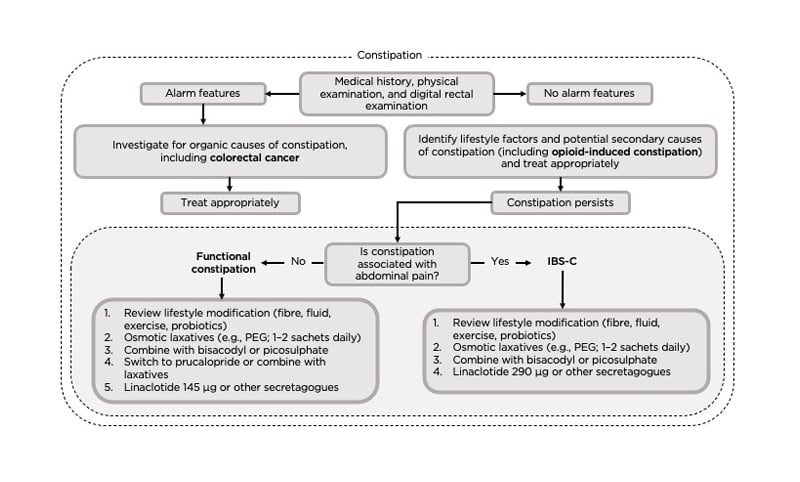
Figure 1: An algorithm for the investigation of patients with constipation.17
DRE: digital rectal examination; IBS-C: irritable bowel syndrome with constipation; PEG: polyethylene glycol.
In addition to the therapies detailed in the treatment algorithm, Corsetti also uses bulking agents as an early line of treatment for constipation. However, Corsetti explained that a study into the effect of bulking agents in different subtypes of constipation found that patients with slow transit and defaecation disorders did not respond to this treatment approach.25 Further studies are needed to investigate the mechanisms of action in different types of constipation in order to apply the best treatment approach for each patient.
One approach to evaluating potential treatment combinations is to investigate their mechanism of action and physiological effects. To investigate the effect of standard constipation medication on colonic motility, Corsetti and her colleagues evaluated the effect of polyethylene glycol, bisacodyl, and prucalopride in healthy subjects (N=10). Polyethylene glycol induced low-amplitude contractions, whereas bisacodyl induced high-amplitude, propagating contractions associated with mass movement. Surprisingly, prucalopride did not induce high-amplitude contractions like bisacodyl, but instead was associated with repetitive, low-amplitude, and simultaneous contractions.26 This suggests that in patients that tolerate prucalopride but are not responding well, bisacodyl could be considered as an adjunct therapy. Corsetti believes that while prucalopride increases the tonic response to gas, bisacodyl induces mass movement, and has seen promising results using the combination treatment approach in patients. Rectal irrigation has also been shown to be effective in several studies27,28 and Corsetti frequently uses this therapeutic approach in patients that do not respond to pharmacological treatment.
After exhausting the available pharmacological therapies for chronic constipation, treatment-refractive patients should undergo anorectal function and gut transit testing to further characterise their condition. Additional treatment options could be considered for functional defaecation disorder such as biofeedback, and surgical options could be discussed if gut transit is abnormal.17
Pancreatic Exocrine Insufficiency and Pancreatic Enzyme Replacement Therapy in Patients with Advanced Pancreatic Cancer: A Systematic Review and Meta-analysis
Enrique de-Madaria
Pancreatic cancer is the second most common type of cancer in Europe and the fourth leading cause of cancer-related mortality.29 The 1-year survival rate for pancreatic cancer is just 24%, and only 9% will survive for 5 years.30 Approximately 30% of patients with pancreatic cancer present with malnutrition, and this is one of the reasons for poor performance status in these patients.31 PEI, which contributes to malnutrition in pancreatic cancer, can result from obstruction of the main pancreatic duct,31,32 and PERT is the standard treatment.33
de-Madaria described a systematic review and meta-analysis that aimed to assess the prevalence and clinical consequences of PEI in patients with advanced pancreatic cancer, alongside the association of PERT with survival and quality of life. Prospective observational studies or randomised controlled trials were selected for the meta-analysis; seven reported the rate of PEI, and seven the effect of PERT (N=673 patients overall).33
The pooled prevalence of PEI in patients with advanced pancreatic cancer was 72% (95% confidence interval: 55–86%), with a high statistical heterogeneity. Two studies addressed the severity of PEI in these patients, showing that 40% of cases were mild-to-moderate, and 17% were severe (according to faecal elastase levels). The pooled risk of PEI was higher for tumours located in the head of the pancreas (56%) compared with the tail (32%), and de-Madaria explained that this was because tumours in the head are more likely to obstruct the main pancreatic duct. The pooled prevalence of diabetes and pre-diabetes in patients with advanced pancreatic cancer was 50% (95% confidence interval: 44–56%).33
In terms of clinical outcomes of PERT, two studies measured the coefficient of fat absorption, and pooled results showed no statistical difference between patients after PERT treatment compared with baseline. Three studies looked at body weight at 8 weeks, and pooled results showed that there was a statistically significant difference of 3.27 kg between patients treated with PERT versus placebo. Six studies assessed survival, with pooled results indicating a statistically significant difference of 3.78 months between groups.33
No evidence of publication bias was identified, but de-Madaria did point out that the meta-analysis was confounded by heterogeneity between studies, small sample sizes, different tests used to diagnose PEI, and regional differences between PEI prevalence. de-Madaria also stressed that it may not be ethical to conduct randomised controlled trials that report overall survival with PERT, since this would require many patients with PEI to remain untreated. Nevertheless, this meta-analysis provides some clues to suggest that PERT may be associated with improved survival in patients with PEI.33
de-Madaria emphasised that treatment adherence by both patients and clinicians has been an issue for PERT in the past, particularly with the large numbers of capsules required for preparations of just 10,000 units of PERT per capsule. More recently, 35,000-unit capsules have become available in some countries, and since these reduce the number of capsules that need to be taken with each meal, they are likely to be associated with improved adherence.
In conclusion, de-Madaria recommended that PERT should be considered as part of the best standard of care in patients with advanced pancreatic cancer as it may prolong survival in patients with PEI, which is present in approximately 75% of patients of this population.
Pancreatic Enzyme Replacement Therapy in Chronic Pancreatitis: Quality of Management and Adherence to Guidelines
Trond Engjom
Further to de-Madaria’s presentation on advanced pancreatic cancer, PEI is also a common complication in patients with chronic pancreatitis.34 UEG guidelines provide clear recommendations for the use of PERT in these patients,34 and Engjom described a study which aimed to assess the quality of adherence to these guidelines and to evaluate the associations of risk factors for, and consequences of, non-adherence.
This was a cross-sectional, observational study using data from the Scandinavian Baltic Pancreatic Club (SBPC) database. PEI was defined as faecal elastase levels <200 µg/g, and a sufficient PERT dose was defined as ≥100,000 lipase units per day. Patients with chronic pancreatitis were included from across eight medical centres (N=1,006).
Over half (64%) of patients were treated with PERT in line with UEG guidelines; however, 25% of patients with PEI were not receiving PERT, and 45% were receiving an insufficient dose. Conversely, 14% of patients who did meet the criteria for PEI were, nevertheless, receiving PERT. Current smoking status was associated with non-treatment in patients with PEI (p<0.001), and both current heavy drinking (>5 units/day), and longer disease duration were associated with receiving insufficient PERT doses (p=0.001). Engjom explained that his team was surprised to find no association between insufficient treatment and underweight or severe vitamin D deficiency, though current smoking status and male sex were associated with these conditions.
Engjom pointed out that there were clear differences in treatment adherence between medical centres within the SBPC database and that this, in association with disparities in the definition of PEI, could contribute to differences in adherence rates compared with recent studies conducted in other regions. In summary, Engjom concluded that even in expert centres that focus on patients with chronic pancreatitis, adherence to UEG recommendations for PERT is insufficient. Prospective studies are needed on long-term adherence and outcomes related to treatment compliance.
MICROBIOME: PRE- AND PROBIOTICS
Targeting the Microbiota in IBS: From Pre- and Probiotics to Faecal Microbiota Transplantation
Gerard Clarke
IBS is considered to be a disorder of gut–brain axis interactions, with cardinal symptoms such as abdominal pain, constipation, and diarrhoea, as well as psychiatric comorbidities, and visceral hypersensitivity.35
Clarke explained that pre-clinical data illustrate that gut microbiota regulate anxiety, depression, and pain, all key features of IBS. For example, faecal transplantation into germ-free animals has shown that gut microbiota can regulate visceral pain,36 anxiety-like behaviours,37–39 the stress response,38 and depression-like behaviour.38,39 In one IBS-specific study, germ-free mice colonised with microbiota from IBS subjects with comorbid anxiety developed both GI dysfunction and anxiety-like behaviour.40
There are a number of different mechanisms through which gut–brain axis function might relate to the features of IBS. Clarke illustrated the example of serotonin, a key signalling molecule in the gut–brain axis that regulates both GI function and central nervous system behaviour; the precursor of serotonin synthesis, tryptophan, has been shown to be regulated by the gut microbiome.39,41
Clarke explained that from a translational perspective, pre-clinical research is supported by cross-sectional studies in human populations, where differences in microbiome composition and function have been observed in both psychiatric disorders and IBS, compared to healthy subjects.42,43
One method to translate pre-clinical findings to clinical data is to isolate potentially beneficial microbial strains identified through pre-clinical studies, and to evaluate them in healthy volunteers. Clarke explained that this approach has yielded positive results in some cases. Bifidobacterium longum 1714, for example, has been shown to attenuate cortisol output following acute stress exposure, and to alter some aspects of brain activity and memory function.44,45 However, this strategy has not been university successful. Lacticaseibacillus rhamnosus JB-1, also identified as a putative beneficial gut microbe through pre-clinical trials, failed to modulate stress or cognitive performance in healthy male subjects.46 Clarke emphasised that the effects of gut microbiota appear to be strain specific, and further research is needed to understand why findings from pre-clinical experiments can be difficult to translate to human studies in this field. Clarke highlighted findings from a systematic review and meta-analysis of randomised clinical trials of probiotics, in adults with IBS, which concluded that although certain microbial species and strains had beneficial effects, their efficacy for IBS remains somewhat unclear.47
Clarke explained that an improved understanding of the gut–brain axis signalling pathways that may be influenced by gut microbiota is allowing researchers to begin to identify candidate strains with specific biological effects such as reducing inflammation or modulating tryptophan metabolism. This information can then be leveraged against the underlying biology of specific patients with IBS;48 for example, there may be some IBS subgroups that are more affected by the immune aspects of the gut–brain axis, while others are better characterised by defects in tryptophan metabolism.








