Meeting Summary
The management of inflammatory bowel disease (IBD) has entered an exciting era, with the optimisation of existing therapies, new strategies being explored that have the potential to further improve patient outcomes, and a growing recognition of the value of a personalised approach to treatment. This symposium explored optimal approaches to using biologic therapy, and the use of therapeutic drug monitoring (TDM) and biomarkers in treatment management.
IBD shows a progressive immunopathogenesis, and a ‘window of opportunity’ exists whereby early intervention may alter the disease course. There is a convincing body of evidence supporting early intervention with anti-TNF-α therapies to improve patient outcomes. Cost is the major barrier to initiating and continuing treatment with biologic therapy. Biosimilars have the potential to reduce costs and increase patient access to biologic therapies, enabling more patients to receive biologic treatment earlier. The use of TDM in the treatment of IBD is increasing and offers benefits over standardised approaches to dosing, and it is likely that emerging dose optimisation tools will enable a personalised approach to treatment in the future.
Many patients experience loss of response to anti-TNF-α therapy. Biomarkers currently used to monitor treatment response include C reactive protein (CRP), faecal calprotectin, and anti-drug antibodies (ADA). Although biomarker identification is still at an early stage for IBD, several genetic, serological, and microbiome markers have also shown promise in predicting response to anti-TNF-α therapy, while other biomarkers are also under investigation for use in diagnosis, predicting response to therapy, and treatment monitoring.
Putting the Pieces Together: When Should Anti-TNF be Introduced?
Professor Stefan Schreiber
IBD shows a progressive immunopathogenesis.1 Following an initial immune response, amplification of this inflammatory response leads to the phenotypic expression of the disease and tissue destruction. In both Crohn’s disease (CD) and ulcerative colitis (UC), T helper (Th) cells contribute to the immune response, which continues to change over time.1 In early IBD, a Th1-driven response is dominant, whereas late IBD is characterised by a mixed Th1/Th17-driven response.1 A better understanding of the pathogenesis of IBD has enabled the development of new therapeutic strategies targeting various inflammatory disease pathways.2
A ‘window of opportunity’ has been identified in which early treatment may have the greatest benefit.3 However, treatment is often started a long time after diagnosis and few patients with IBD are initiated on biologic therapy. An analysis of IBD treatment pathways in the USA found that only 3.9–4.1% of patients with CD and 0.5–0.8% of patients with UC initiated treatment with a biologic,4 with the most commonly used first-line therapies being corticosteroids (42% of patients with CD) and 5-aminosalicylic acid (35% of patients with CD and 59–64% of patients with UC). Early intervention with biologics is not appropriate for all patients and it is important to balance the potential for benefit with the risk of experiencing side effects. Prognostic factors can be used to help select patients who may benefit the most from early intervention. In patients with CD, prognostic factors for disease progression include ileal disease location, perianal disease, upper gastrointestinal involvement, and extraintestinal manifestations, together with younger age, smoking, endoscopic severity, serological reactivity to certain microbial antigens, and genetic mutations (although genetic markers are not routinely used in current clinical practice).5
There is a growing body of evidence supporting early intervention with anti-TNF-α, with greater treatment benefits evident in patients with a shorter duration of disease.6-8 In the PRECiSE 2 trial in CD, patients (N=425) who responded to induction therapy and received certolizumab pegol were randomised to maintenance treatment with certolizumab pegol or placebo for 26 weeks. In the cohort treated with certolizumab pegol, response and remission rates were higher in patients with a disease duration of <1 year compared with those with a disease duration of ≥5 years. In contrast, no association between efficacy and disease duration was seen in patients treated with placebo.6 In the CHARM and ADHERE studies, patients with moderate-to-severe CD were divided into three disease duration categories (<2 years, n=93; 2 to <5 years, n=148; and ≥5 years, n=536) and treated with adalimumab or placebo. Through all time points up to Week 164, remission rates were numerically higher in patients with disease duration of <2 years, compared with those in the longer disease duration groups. Logistical regression analyses found that remission at Week 56 was significantly associated with shorter baseline disease duration (p=0.046).7
In patients with UC, early success of therapy may be more important than disease duration, as rapid mucosal healing is a strong prognostic factor associated with improved outcomes.8 In the ACT-1 and ACT-2 studies in patients with moderate-to-severe UC who received infliximab or placebo, achievement of mucosal healing at Week 8 was associated with improvements in time to colectomy, rates of symptomatic remission, and corticosteroid-free symptomatic remission. Furthermore, patients with a greater degree of mucosal healing at Week 8 were most likely to sustain mucosal healing to Week 54.8
The 2-year, open-label ‘Top-Down versus Step-Up’ trial was conducted in patients with active CD (N=133) randomised to early combined immunosuppression (infliximab plus azathioprine; ‘top-down’) or conventional treatment (‘step-up’).9 At Weeks 26 and 52, significantly more patients in the ‘top-down’ versus the ‘step-up’ group achieved remission. Even after 8 years, a difference between the two groups was evident in key endpoints important in the natural course of the disease. Compared with conventional step-up treatment, top-down treatment resulted in a reduced proportion of patients experiencing a flare and a longer median time to flare, although there was no significant difference in remission rates.10 In the REACT trial, patients with CD received early combined immunosuppression (ECI; adalimumab or infliximab and azathioprine, 6-mercaptopurine or methotrexate) or conventional management (according to the centre’s usual practice). In the ECI group, the presence of active disease resulted in dose escalation of the anti-TNF-α therapy. At 24 months, patient-level composite rate of surgery, hospital admission, or serious disease-related complications was lower for the ECI group than the conventional management group (hazard ratio [HR]: 0.73; p=0.0003).11
In the past, cost has been the major barrier to initiating and continuing treatment with biologics.12 Biosimilars have the potential to reduce costs and increase the number of patients able to access biologic therapies, making early intervention a realistic option. In the UK, cumulative cost savings from the introduction of infliximab and etanercept biosimilars were £38.8 million between 2015 and 2017.13 In 2015, in Norway, the prices of infliximab biosimilars were lowered to 64% of the cost of the reference product, resulting in a 34% increase in infliximab use by the following year.14 The potential 1-year budget impact of introducing biosimilar infliximab on direct drug healthcare costs was modelled in five European countries (Belgium, Germany, Italy, the Netherlands, and the UK). A scenario in which the cost of the biosimilar was 30% lower than that of the reference product would equate to an annual saving of €35.9 million in patients with CD and €15.4 million in patients with UC, allowing 3,309 and 1,392 additional patients with CD or UC, respectively, to be treated each year.15 Despite these clear benefits, the uptake of anti-TNF-α biosimilars varies widely across Europe (0–65% of market share).16
Prof Schreiber concluded that early intervention with anti-TNF-α slows disease progression and improves long-term outcomes in patients with IBD. Biologic therapy is a major cost-driver in the management of IBD, but the use of biosimilars can reduce costs and expand access, enabling more patients to receive earlier treatment.
Solving the Riddle: How Do We Implement Therapeutic Drug Monitoring to Maximise Treatment Success?
Professor Walter Reinisch
There is growing evidence for the use of TDM in IBD, whereby measurements of anti-TNF-α drug levels and antibodies against the TNF inhibitor are used to tailor therapy. This offers potential benefits over a standardised approach to dosing, enabling personalisation of therapy together with the ability to monitor treatment compliance, observe changes in pharmacokinetics (PK), reduce drug toxicity, optimise outcomes, increase cost-effectiveness, and define the biosimilarity of biologics.
Considerable interpatient variability in pre-infusion drug concentration has been observed with biologics, including infliximab and adalimumab.17,18 Drug clearance over time and drug serum levels are key PK parameters in patients with IBD.19 Various factors can affect these parameters, which may impact on treatment outcomes with anti-TNF-α therapies.19 These factors include body weight/BMI, the development of ADA, inflammatory burden, serum albumin levels, and the use of immunomodulators (Figure 1).19
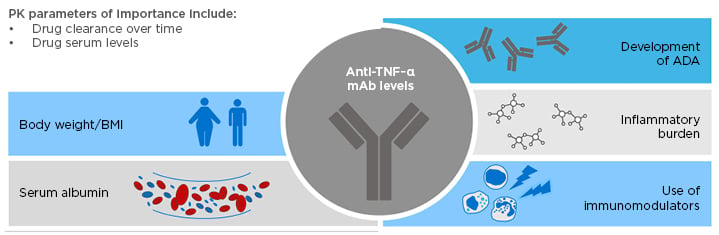
Figure 1: Factors affecting pharmacokinetic parameters in patients with inflammatory bowel disease, which may impact on treatment outcomes with anti-TNF-α therapies.
ADA: anti-drug antibody; IBD: inflammatory bowel disease; mAb: monoclonal antibody; PK: pharmacokinetic.
However, these PK parameters do not appear to differ between patients treated with adalimumab reference product versus a biosimilar (ABP 501). In a 26-week, randomised, double-blind, active comparator-controlled equivalence study of patients with moderate-to-severe active rheumatoid arthritis despite methotrexate, drug trough serum concentrations were similar in patients treated with either reference adalimumab or adalimumab biosimilar ABP 501 across all time points to Week 26.20 The development of binding and neutralising ADA over time was also similar in patients treated with ABP 501 and those who switched from reference adalimumab to ABP 501.21
There is a clear exposure–response relationship for adalimumab, with higher drug serum concentrations during both induction and maintenance being associated with increased rates of remission.22,23 In patients with UC treated with adalimumab, the probability of remission at Week 8 increased with increasing serum adalimumab trough concentrations.22 In patients with CD, both adalimumab drug and ADA levels are predictors of clinical response.23 A cross-sectional study showed that serum adalimumab levels above 5.85 μg/mL and ADA levels below 1.50 µg/mL–eq were associated with highest likelihood of remission.23
The use of TDM in the treatment of IBD is becoming increasingly common, and the current view is that proactive and reactive TDM should be considered as complementary, and not mutually exclusive, strategies.24 Reactive TDM can potentially be used when treatment failure occurs,25,26 to confirm that symptoms are caused by the disease, and better direct and guide patient care.25,27 Proactive TDM may be used during induction, maintenance, or remission,24,25,28 or in therapy de-escalation (i.e., stopping or reducing dose).24,28 Compared with reactive TDM, a proactive approach is associated with lower rates of ADA, treatment failure, and IBD-related surgery or hospitalisations.27 Data regarding the role of TDM during anti-TNF-α induction therapy in IBD are limited,26 and TDM is usually applied in the maintenance setting. In a retrospective study in patents with IBD (N=264) who received infliximab maintenance therapy, proactive drug monitoring was associated with improved clinical outcomes compared with reactive monitoring, with a reduced risk of treatment failure (HR: 0.16; 95% confidence interval [CI]: 0.09–0.27; p<0.001), IBD-related surgery (HR: 0.30; 95% CI: 0.11–0.80; p=0.017), and IBD-related hospitalisation (HR: 0.16; 95% CI: 0.07–0.33; p<0.001).27
Prof Reinisch described a dose-optimisation tool that has recently been developed using a predictive PK algorithm based on Bayesian modelling.29 The physician is required to enter patient factors, select a target treatment dose, and choose the dose and dosing interval via a cloud-based clinical dashboard. Using this approach, three drug serum concentration measurements can provide sufficient information for individualised dose adjustment in patients with IBD. Assessment of long-term treatment retention showed that patients dosed in accordance with the dashboard’s recommendations were more likely to remain on treatment over a follow-up period of 72 months.29
Looking ahead to the potential future role of TDM in IBD, it is likely that emerging dose-optimisation tools will allow a personalised approach to treatment. TDM may enable earlier identification of patients who do not have a clinical response and could provide early insights into resistance and response, which may help to profile patient populations more likely to respond to anti-TNF-α therapy and help to ensure patients receive the optimal drug dose.
Unravelling the Problem: How Can Biomarkers Be Used to Guide Clinical Management?
Doctor Gionata Fiorino
The aetiology of IBD is multifactorial, with genetic factors, lifestyle, medication, intestinal microbiota, and mucosal immunology all contributing to the heterogeneity of the disease.30 Molecular profiling has the potential to define disease heterogeneity, identify relevant biomarkers, and ultimately stratify patient subsets who can then receive personalised healthcare in the form of disease-specific therapeutic agents. Patients in a given subset will likely still differ, but treatment with therapies targeted towards core pathways has the potential to enrich the therapeutic response.31 IBD represents a group of patients with inherently variable disease courses. There is a changing biology from early to late stages of IBD, with the inflammatory response composed of an early Th1-driven and a late Th17-driven inflammatory response, and TNF-α produced mainly in the early stages of the disease.1 Differences exist between patients in their inflammatory profiles due to differing disease stage (i.e., early versus late), endoscopic activity, and disease location. Understanding the expression of biomarkers across the disease course will help in selecting the most appropriate patients and targets for therapy.
Biomarker identification is still at an early stage in IBD. Biomarkers in clinical use and in development differ in their complexity and clinical utility (Figure 2).32-38 Protein biomarkers such as CRP, faecal calprotectin, ADA, and drug trough levels show a low level of complexity, and are currently used in clinical practice to monitor inflammation and guide treatment strategy decisions.32-34 Other more complex types of biomarker, such as mRNA, DNA, and the microbiome are currently under investigation, and could allow the identification of patients who may respond to a particular therapy.
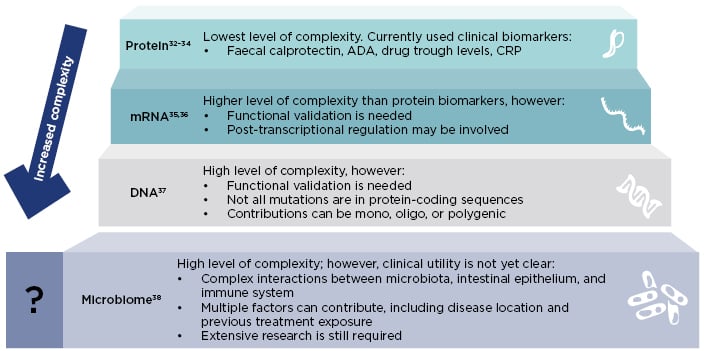
Figure 2: Different types of biomarkers have different complexity and clinical utility.
ADA: anti-drug antibody; CRP: C-reactive protein.
Anti-TNF-α therapy is not effective in all patients with IBD,39-42 and many patients experience loss of response to therapy in the long term.43 In case series and randomised controlled trials conducted in patients with CD, the rate of loss of response at 12 months ranges from 23% to 46%.43 Biomarkers currently used to monitor response to treatment with adalimumab include CRP, faecal calprotectin, and ADA.26,44,45 CRP is a standard marker for the acute phase response across inflammatory diseases,46 and can be used as a surrogate marker of active CD and an indicator of treatment response.47 A decrease in CRP level is indicative of response to therapy, while persistently high levels of CRP are associated with diminished or loss of response.47 Serum CRP levels may therefore be useful for assessing a patient’s risk of relapse. However, CRP is a less powerful biomarker than faecal markers and patients with normal levels of CRP may have endoscopically active disease.47 Decreasing levels of faecal calprotectin appear to correlate with clinical response and mucosal healing. Faecal calprotectin can help predict relapse and postoperative recurrence, and is useful in determining when, and in whom, a more invasive endoscopy should be performed. Faecal calprotectin has reduced value in patients with CD restricted to the small bowel, and shows an imperfect correlation with transmural inflammation.47
The presence of ADA is associated with negative therapeutic outcomes. There is a relationship between the presence of ADA and sub-therapeutic drug concentrations, and lower or undetectable drug concentrations are associated with treatment failure.26 Neutralising ADA (a subset of binding ADA) can inhibit drug activity, while binding ADA can increase drug clearance.45 Biosimilars show similar immunogenicity profiles to their reference biologics. In a 26-week randomised, double-blind equivalence study in which patients with moderate-to-severe active rheumatoid arthritis despite methotrexate received ABP 501 or reference adalimumab (40 mg) every 2 weeks, a total of 38.3% and 38.2% of patients, respectively, tested positive for binding ADA, while 9.1% and 11.1% were positive for neutralising ADA.20 There is limited guidance on the optimal use of ADA in treatment management and limited availability of accurate, rapid, easily administered, and inexpensive tests.26,48
Biomarkers may be valuable when using a treat-to-target approach, which involves predefining a treatment target associated with optimal long-term outcomes in consultation with the patient, then continuously monitoring disease activity and modifying treatment until the target is reached. All components (i.e., target, treatment, and monitoring) are tailored to the needs of the individual patient, and de-escalation of therapy may be considered when treatment goals are achieved.49,50 CRP and faecal calprotectin are the best surrogate markers currently available for assessing endoscopic activity. The randomised, controlled CALM study demonstrated that a treatment algorithm based on these biomarkers, in conjunction with the Crohn’s Disease Activity Index (CDAI) and prednisone use, resulted in better clinical and endoscopic outcomes (i.e., a greater proportion of patients achieving mucosal healing [CDAI <4] and no deep ulcers on endoscopy, deep remission, and biological remission) than symptom-driven decisions alone in patients with active, endoscopic CD, and can be used to guide treatment decisions.51
Several biomarkers have shown promise in predicting anti-TNF-α response. These include genetic biomarkers (e.g., polymorphisms in FCGR3A, TLR2, TLR4, TLR9, TNFRSF1A, IFNG, IL6, and IL1B), serological biomarkers (e.g., pANCA, haemoglobin, serum albumin, and TREM-1), and microbiome biomarkers (e.g., Faecalibacterium prausnitzii in UC).52-54 Various other biomarkers are also under investigation for use in diagnosis, predicting response to therapy, or in treatment monitoring (Figure 3).54-59
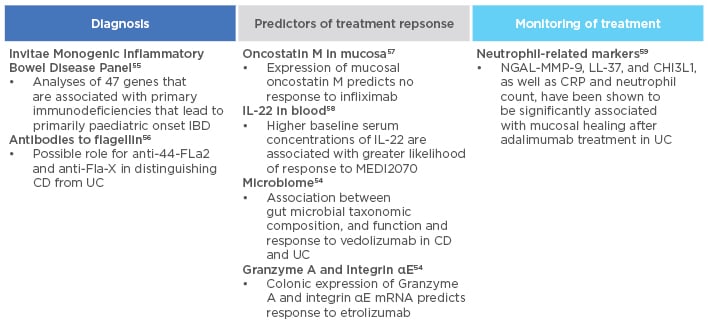
Figure 3: Biomarkers under investigation in inflammatory bowel disease.
CD: Crohn’s disease; CRP: C-reactive protein; IBD: inflammatory bowel disease; LL-37: cathelicidin; MMP: matrix metalloproteinase; NGAL: neutrophil gelatinase-associated lipocalin; UC: ulcerative colitis.
In the future, it will be valuable to identify biomarkers that will help to find patients at risk of developing disease, disease progression, or complications, and to determine the right time for biologic treatment, guide treatment decisions, and select the most appropriate drugs for each individual patient.
Concluding Remarks
A growing body of evidence supports early treatment with anti-TNF-α to slow disease progression and improve long-term outcomes in patients with IBD. However, cost is the main barrier limiting the use of biologic therapy. Biosimilars can reduce costs and facilitate increased access, enabling more patients to receive biologic treatment earlier. The use of TDM in the treatment of IBD is increasing and may enable earlier identification of patients without clinical response and help to profile patient populations more likely to respond to anti-TNF-α therapy. The future availability of dose optimisation tools also has the potential to enable a personalised approach to treatment. Biomarkers play a key role in the management of IBD. CRP, faecal calprotectin, and ADA are currently used to monitor response to anti-TNF-α therapy, and several other genetic, serological, and microbiome markers have shown promise as predictors of response. The search will continue for novel biomarkers to identify patients at risk of disease, determine the optimal time for biologic treatment, and guide treatment decisions.
WATCH THE FULL WEBCAST OF THE SYMPOSIUM ONLINE
https://vimeo.com/330729181
Enter the password “ECCO2019” when prompted








