Chairperson: Frédéric Gottrand1
Speakers: Claudio Romano,2 Frédéric Gottrand,1 Valérie Marchand3,4
1. Department of Pediatric Gastroenterology Hepatology and Nutrition, Lille University, Lille, France
2. Pediatric Gastroenterology and Cystic Fibrosis Unit, University of Messina, Messina, Italy
3. Department of Pediatrics, University of Montréal, Montréal, Canada
4. Pediatric Gastroenterology, Centre Hospitalier Universitaire (CHU) Sainte-Justine, Montréal, Canada
Disclosure: Prof Gottrand has received grant/research support from Lactalis; and has served as a member of an advisory board for Nestlé Clinical Nutrition. Prof Romano has received consultancy fees from AbbVie, Nestlé, and Nutricia. Prof Marchand has participated in speakers bureau for Abbott, Nestlé, and Takeda Pharmaceutical Company.
Acknowledgements: Medical writing assistance was provided by Dr Eleanor Roberts, Beeline Science Communications, Ltd, London, UK, on behalf of EMJ.
Support: The publication of this article was funded by Nestlé Health Science. Content was reviewed by the speakers for medical accuracy.
Citation: EMJ. 2020;5[4]:29-38.
Meeting Summary
Due to feeding and digestion difficulties, children with cerebral palsy (CP) can be at risk of malnutrition. European nutritional guidelines regarding children with neurological impairment (NI) have stressed the importance of identifying nutritional difficulties through factors beyond weight and height, such as assessment of fat mass, bone mineral density, and nutritional status. Feeding difficulties can be caused by a combination of oral- and gut-related problems, such as postural complications, swallowing difficulties, and gastro-oesophageal reflux disease (GORD). If oral feeding is too difficult, or is unsafe, a feeding tube may need to be inserted either into the stomach or jejunum. Once the feeding method is established, other considerations include ensuring energy needs are being met. These must be individually assessed because of large differences in energy needs and body composition. One way such needs can be met is through the use of formulas with adequate caloric and nutritional values; another is by using blenderised food, tailored to the individual’s dietary needs and preferences. Further gastrointestinal problems include diarrhoea and constipation, which may also be helped with a blenderised food diet and/or with addition of dietary fibre to formulas. Such nutritional management of children with CP involves a multidisciplinary team of healthcare professionals, the child, and their family. During this symposium, Prof Romano, Prof Gottrand, and Prof Marchand discussed findings from their own practices, professional guidelines, and clinical studies that can aid in identifying nutritional deficiencies and managing the nutritional needs of children with CP.
Overview
CP, the most common NI in children, is caused by nonprogressive damage or malformation while the brain is developing. The manifestation of CP is heterogenous and can affect an individual’s speech, motor skills, vision, memory, muscle actions, and learning abilities.1
While advances in supportive care, which have mainly focussed on respiratory and orthopaedic problems, have extended the life expectancy of people with CP, what also needs to be addressed is another problem experienced by patients: chronic undernutrition or malnutrition.2 In this series of three talks, Prof Romano discussed ‘Guidelines and importance of fibres in paediatric enteral nutrition [EN],’ Prof Gottrand talked about the ‘Importance of percutaneous endoscopic gastrostomy [PEG] and patient’s energy needs: how and which formula to use,’ and Prof Marchand provided practical advice on the use of blenderised food in her talk, ‘Use of real food in real life.’
The Problem of Nutrition and Malnutrition in Children with Cerebral Palsy
Assessment of Nutritional Status
A recent study of 325 children with CP found that approximately 75% were underweight, 50% had dysphagia, and >40% had more than one risk factor for malnutrition.3 These findings suggested that nutritional needs and feeding challenges of all children with CP should be assessed regularly. The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Guidelines working group (ESPGHAN-WG), of which Prof Romano was the lead, has provided in-depth guidelines for evaluating and treating gastrointestinal and nutritional complications in children with NI.4
Assessment of undernutrition/malnutrition in a child with CP should not be based on CP-specific growth charts as, discussed Prof Gottrand, “CP children often have a low body size [both height and weight] and [a different] composition from neurologically developed children.” Instead, according to the ESPGHAN-WG, signs of malnutrition include a weight for age that is â¥2 standard deviations below the mean and fat mass measurements (triceps skin fold, arm muscle area) below the 10th percentile. Assessment should also include measurement of bone mineral density and micronutrients, as well as being vigilant of other warning signs of malnutrition, such as skin problems associated with prolonged sitting or lying, poor peripheral circulation, and failure to thrive.4
While the digestive system of a child with CP may be physiologically normal, receiving adequate nutrition can be impacted by many different factors. These include oral problems, such as swallowing difficulties, drooling, and dental abnormalities; postural difficulties caused by pain, spasticity, and hip luxation; and digestive problems, such as GORD, oesophageal motility, vomiting, aerophagia, delayed gastric emptying, and constipation.2,4,5 GORD is a particular problem in children with CP that can be associated with poor feeding.5,6 Investigations for this condition include endoscopy, intraluminal impedance and pH-metry, and biopsy. If these are difficult to perform, the ESPGHAN-WG have suggested an initial trial of proton pump inhibitors (PPI).4
Feeding difficulties can also arise as a result of factors such as a lack of appetite, cognitive problems, depression, and medication side effects.2,4,5 “This is important to keep in mind,” discussed Prof Gottrand, “because some of these factors have therapeutic options. Correcting dental abnormalities, treating pain, treating depression, limiting drug side effects, or treating GORD could all be tried rather than going directly to tube feeding or a gastrostomy.”
Energy Needs of Children with Cerebral Palsy
One goal of adequate nutrition is to meet a person’s energy needs. The ESPGHAN-WG recommend using dietary reference standards for typically developing children to gauge the number of calories needed by a child with CP.4 Approximately 70-75% of energy expenditure, explained Prof Gottrand, “is explained by basal metabolic rate, which is strongly influenced by body size and composition, especially fat-free mass.” As such, it was also noted that reference standards may overestimate energy needs because of the low weight and height of many children with CP and variation in muscle tone.4
Energy requirements may also vary owing to level of disability and ambulatory ability. For example, energy expenditure was examined in one study with regard to the Gross Motor Function Classification System (GMFCS), which ranges from Level I, where someone can walk without restriction, to Level V, where self-mobility is severely limited.7 In the study, in those who could ambulate to any degree (Levels I-IV), a significantly higher amount of energy was needed to perform the same walking task as someone without any disability when each level was compared with the one below.8
Interventions for Nutritional Deficiencies in Children with Cerebral Palsy
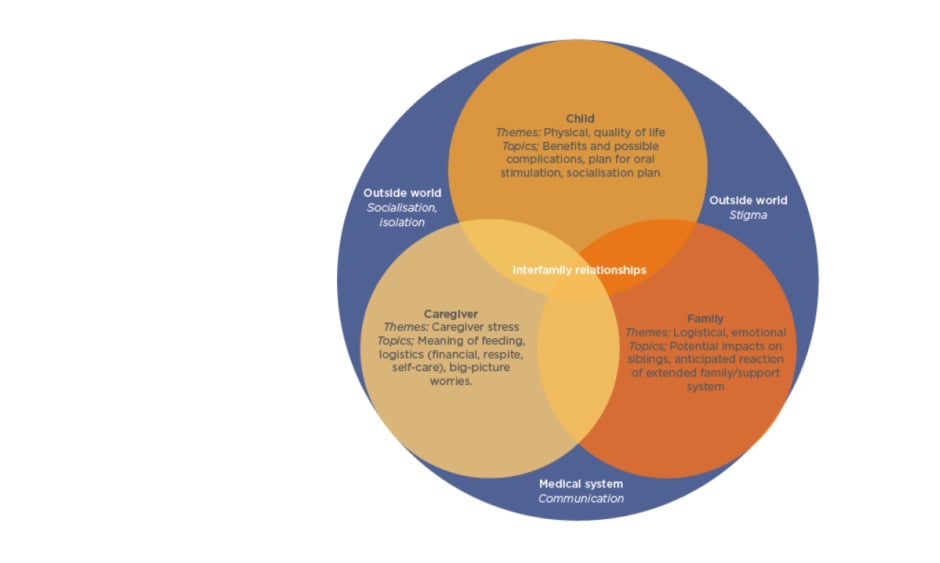
For children with CP, oral feeding can be considered as long as it is safe, nutritionally sufficient, stress-free, and takes no longer than 3 hours a day. However, dental problems, postural difficulties, and orthopaedic issues that can contribute to oral feeding hazards mean that while oral nutritional intake may be adequate, it may not be considered safe on account of dangerous occurrences, such as pulmonary aspiration (Figure 1).4
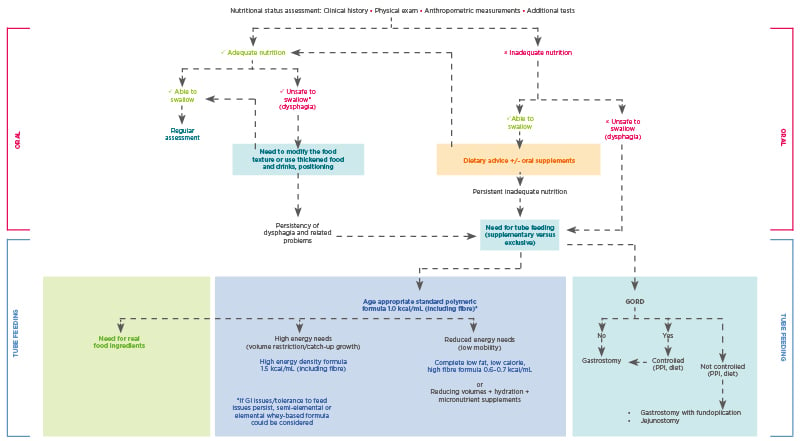
Figure 1: Nutritional management of children with neurological impairment.
Unsafe swallowing is defined as a history of aspiration pneumonia (antibiotics or hospital admission for chest infection) and objective evidence of aspiration or penetration on contrast videofluoroscopy.
GORD: gastro-oesophageal reflux; GI: gastrointestinal; PHGG: partially hydrolysed guar gum; PPI: proton pump inhibitor.
Adapted from Romano et al.4
Click here to view larger.
Prof Romano discussed how there are some very basic ways to help a child with CP gain adequate nutrition, such as making sure their seating posture when eating is optimal, providing food of a consistency that is easy to swallow, and making sure the caloric density and fibre content of meals is adequate.4
Importance of Dietary Fibre
Prof Romano focussed on the beneficial effects of dietary fibre. Sources of fibre in enteral formulas include those that are soluble, such as pectin and guar, which are fermented to short-chain fatty acids by colonic bacteria and provide fuel for large intestine endothelial cells, and those that are insoluble, such as soy polysaccharide, which increases faecal weight and colonic peristalsis.9,10 Another use of dietary fibre is for prebiotic needs thanks to its resistance to gastric activity, enzyme hydrolysis, and gastrointestinal absorption. Fibres can also stimulate growth and activity of ‘healthy’ intestinal bacteria, such as bifidobacteria.11
Recent EN guidelines from the European Society for Clinical Nutrition and Metabolism (ESPEN) recommended, in adult and paediatric populations, fibre-containing enteral feeds, particularly when a person is experiencing diarrhoea or constipation.12 In support of this, a study in which a standard enteral diet was enriched with 20 g/L of partially hydrolysed guar gum found that diarrhoea occurrence was significantly reduced compared with the standard diet alone.13 Further, two meta-analyses in adult populations confirmed a reduction in diarrhoea or constipation incidence when an enteral formula included fibre.10,14
During the discussion, Prof Romano suggested that soluble fibre was preferred to alleviate diarrhoea, although “it is viscous and can be difficult [to administer] when using a gastrostomy and tube feeding.” Prof Gottrand highlighted: “As many CP children don’t move, constipation is frequent and hard to treat and they need high quantities of laxatives.” He discussed how the amount of fibre included even in fibre-enriched formulas may be too low to correct gastrointestinal problems, including constipation, and thus would need enhancing. As such, all speakers agreed that â¥10 g/day of extra fibre may be needed, especially in nonambulatory children, although studies have not been carried out to ascertain optimal fibre intake.
Enteral Nutrition
For those unable to gain enough nutrition through oral feeding, EN via a PEG tube may be required to supplement or replace the oral method.4 If problems such as GORD-related aspiration, refractory vomiting, retching, and bloating occur with PEG feeding, the ESPGHAN-WG suggested using jejunal feeding.4
Prof Gottrand stressed “the crucial importance in taking parents/caregivers on board when discussing the process of gastrostomy feeding.” The decision whether to use EN has to be made by assessing the needs of not only the child, but also their parents/caregivers and the family as a whole (Figure 2). For the child, important considerations include both physical aspects of EN, including benefits and complications, social aspects of how PEG feeding fits in with their daily routine, and quality of life.
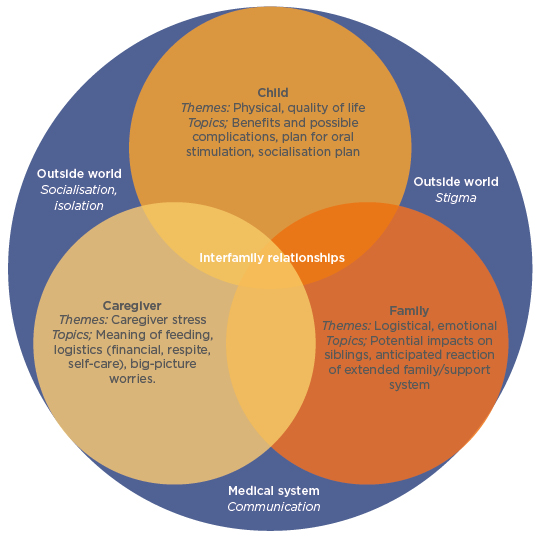
Figure 2: Themes and topics to be considered during discussions regarding placement of a gastrostomy tube.
Adapted from Nelson et al.15
For the parent/caregiver, there are challenges regarding how PEG feeding is carried out, logistical considerations including financial needs, and awareness of how much stress PEG feeding may cause. For the family, considerations may include how PEG feeding fits into mealtimes, as well as emotional aspects such as impact on siblings, and whether wider family support is available. Considerations outside of the family unit include stigma, socialisation, and communication within the medical system.15
The primary benefit of EN is that nutritional status and growth can be improved.16,17 For example, in a study of 368 children/young adults (age: 1 month-25 years old) tube fed via PEG, a significant weight and height catch-up was observed over a median follow-up of 2.4 years.17 Other benefits include alleviation of oral feeding problems, such as coughing and choking, and improvement in quality of life for the parents/caregivers.18
The type of EN used depends on a number of factors, such as the child’s age, their energy requirements, and the mode of enteral access. It may, according to Prof Gottrand, be given as a combination of daytime bolus feeds and nocturnal continuous feeds, especially in children with high-caloric needs or poor tolerance to high volumes of food in one sitting. EN will often be in the form of a commercially available formula, and the ESPGHAN-WG have suggested that a standard (1.0 kilocalorie [kcal]/mL) polymeric age-appropriate formula including fibre is adequate for most children past their first year. In those who cannot tolerate a high volume of food, a high-energy density formula (1.5 kcal/mL) may be required. The ESPGHAN-WG also recommended at least yearly checking of protein and micronutrient intake compared to dietary reference standards, with supplementation if needed.4
During EN feeding, some assessments need to be regularly carried out, such as monitoring of body weight and fat mass. Additional nutrition may be needed for ‘catch-up growth’; however, once this is achieved there is a risk of over-feeding, leading to obesity.4 In the discussion, it was asked whether over-feeding can be diminished by reducing formula volume administered or by diluting it. “Though this is possible,” Prof Romano remarked, “the risk is that [reducing the amount] won’t cover the volume needs of these children as many don’t experience thirst so are chronically under-watered.” Dilution also has risks, he continued, as this could “reduce the micronutrient and fibre intake, which is often low as energy need is low.”
In children who are immobile, low energy needs suggest a low-fat, low-calorie, high-fibre, and micronutrient-replete formula may be needed.4 Assessment of this in one study of 14 mostly immobile children with CP (age: 10 months-11 years old) who had received a PEG found that after 6 months, adequate nutritional status and growth, without excess weight, was achieved despite the fact that the formula was 50% of the estimated average energy requirement for children of their respective ages.19 In the discussion, it was highlighted that as formula volume may be very low in those with low energy needs, it is important to maintain fluid levels because, according to Prof Gottrand, problems such as kidney stones could arise. Prof Marchand pointed out that a practical way to overcome this is to include fluid rinses after feeding and medication administration. Another problem related to these low calorie EN formulas that arose during the discussion was that they are unfortunately not available in some countries and/or are not reimbursable via the healthcare system.
As with oral feeding, GORD can be a problem when feeding via a PEG (Figure 1). In a study of 326 children/young adults with a newly-situated gastrostomy (age: 1 month-25 years old), 12% experienced new incidences of GORD and it was exacerbated in 25% of the 74% who already experienced it.20 ESPGHAN-WG suggestions for treating EN-related GORD include thickening the formula, using a whey-based formula, and using a PPI.4 Another solution is antireflux surgery, as was required by 16% of those with GORD in the above study.20 During the discussion, when asked whether different nutritional therapies were needed for those who had undergone similar surgery, Prof Gottrand said that while there were few comparison studies, in his experience there is no clinical advantage of any one formula type.
Blenderised Tube Feeding
Before 1970, reported Prof Marchand, tube feeds often consisted of blenderised food. While commercial formulas took over for a period of time, there has been a growing trend among families and dietitians to revert to blenderised food. A recent survey of blenderised tube feeding (BTF) found over one-half of 54 adult patients using EN used BTF.21 In another survey, nearly 90% of 125 children who required long-term EN were administered BTF for a mean of 71% of their total daily nutritional intake.22 Additionally, a survey of registered dietitians found that 58% used BTF, with most agreeing the experience was positive for the family, child, and the clinical practice.23
The use of BTF depends on a team of people including the child’s physician, to assess medical suitability; a registered dietitian, to help devise recipes using advanced nutrition analysis software; and caregivers, to evaluate daily aspects of BTF administration. These people need to work together, stressed Prof Marchand, to assess these aspects both prior to initiation and throughout.24 Two recent examinations of how best to start with BTF highlighted a variety of aspects for consideration.24,25 The patient should be medically stable on EN with a mature gastrostomy tube of at least size 12 French, they should have the ability to tolerate bolus feeding, and medical and family support should be adequate for both initiation and maintenance, with the right equipment and ability for food preparation and storage.25 Prof Marchand also pointed out some contraindications, such as in babies <6 months old, patients in intensive care, individuals who are fed via a nasogastric or nasojejunal tube, and in children who require continuous feeds.
With home-made blenderised foods, it is important that the medical team evaluate the child’s nutritional needs so that personalised recipes can be provided. This is important because a study of 433 parents of children who were tube fed found that 49.5% of those who used blenderised food did so without any professional guidance.26
Blenderised meals usually include a liquid base, such as milk or formula; a protein source; grains; fruits; vegetables; and oil, with micronutrients, sodium, and water added as required.27 Prof Marchand advised that recipes also need to account for local and seasonal food availability, variety, and limiting potential environmental toxins, such as mercury in fish and arsenic in rice. Once prepared, blenderised food must be refrigerated, but must be warmed prior to administration. BTF is delivered as a bolus and should be administered in <2 hours. During the discussion, Prof Marchand highlighted how BTF is not exclusive and can be combined with night-time feeds with formula. Caregivers need to be taught many other aspects of blenderised food preparation as well as composition, including portion size, correct measuring techniques, how to read food labels for nutritional value, and how to clean and store food preparation utensils.24
The advantages of blenderised food, reported Prof Marchand, include that it can be tailored to individual nutritional and micronutritional needs, such as specific food allergies and intolerances. It may also be perceived as being more natural than commercial formulas, and “gives the parents a feeling of normalcy,” helping them feel nurturing and in control of their child’s nutrition.26 Feeding and digestion-related advantages include an increase in oral intake and a decrease in gagging,28 improvements in diarrhoea and vomiting,29 and microbiome diversity.30 Prof Marchand also highlighted how, in her experience, “the use of a real food blend is also the most effective way to address reflux, better than an elemental or semi-elemental diet.” Additionally, blended food may be less expensive than commercial formula if the latter is not covered by a person’s healthcare provider.25
To examine the use of blenderised food, the 6-month ‘BLEND’ study included 20 children (age: 1-16 years old) with a PEG who, at baseline, used commercial formula for 75% of their calorie intake. Caregivers were provided with instructions for prescription formulas taking into account their child’s calorie, liquid, portion, and sodium needs. It was noted in this study that 50% more calories than usual feeds were needed to maintain a stable BMI with blenderised food, although micronutrient intake was similar or better. Mean energy intake, which at the beginning of the study was 74 kcal/kg, increased to 111 kcal/kg with blenderised feeds.30
After 6 months, there was an increase in the number of children with a triceps skin fold measurement >5th percentile, from 76% to 82%, and in the percentage of participants who consumed something by mouth (67% versus 80%). Vomiting more than once a week decreased (76% versus 53%) and gagging/retching also decreased (82% versus 47%). There was a decrease in the number of children needing antacid medication (88% versus 76%), and an increase in those who required a stool softener (24% versus 29%). Significant changes were found in microbial diversity and richness in stool samples (Figure 3). Importantly, the study also found that most caregivers (94%) agreed that BTF was successful and that their child appeared in better health and was happier. All caregivers said they would recommend BTF.30
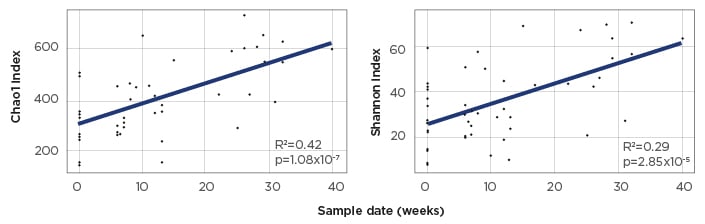
Figure 3: α diversity (Chao1 index) and microbial richness (Shannon index) of microbiota from stool samples with the BLEND diet.
α diversity indices were calculated using data rarefied to 50,000 reads per sample. Statistical significance of increased species diversity and richness was calculated using linear regression with p<0.05 considered significant.
Adapted from Gallagher et al.30
As examples of successes with blenderised food, Prof Marchand described her first experiences, both of which involved 4-year-old children with a PEG whose families had tried multiple different formulas and feeding regimens. For the first child, who had a very short bowel, the main problem was severe diarrhoea and bacterial overgrowth. The child was able to feed orally, though intake was limited. As a result of BTF, the child’s diarrhoea decreased; there was less bacterial overgrowth; decreased need for antibiotics; improved nutritional status; and, eventually, she was weaned off the PEG and all blenderised food was administered orally. For her second patient, who did not feed orally, the main problems were inability to gain weight, vomiting, and GORD. The latter was unresolved with prokinetics and PPI, therefore antireflux surgery was considered. Following the use of BTF, the child gained weight, the vomiting resolved, and surgery was avoided.
While there are several advantages to blenderised food, Prof Marchand also discussed some disadvantages. For example, the composition of blenderised food is not standardised; a larger volume is required; it requires bolus feeding, often with a syringe that can quickly wear out; tubes can become obstructed; it can be time consuming; and it may cost more as it will not be covered by medical insurance. Prof Marchand highlighted commercially available, real food-based formula as a convenient and efficient alternative. Prepared food may be more liable to contamination than commercially prepared EN; however, a comparison between blenderised food and standard polymeric formula and a BTF made using commercial baby food found no difference in bacteria content after being left for 2-4 hours.31 It remains very important to stress to parents that blenderised food should only be used in bolus or short-time infusion to avoid the risk of bacterial contamination.
In conclusion, Prof Marchand stressed that “we, as healthcare professionals, need to keep an open mind and see the benefits [of BTF]. We need to support parents in their quest to provide their child with the best and provide them with guidance to do it in a safe manner with adequate nutritional follow-up.”
Conclusion
The high prevalence of undernutrition, growth impairment, poor body mass density, and micronutrient deficiencies in children with CP means ongoing assessment and monitoring of nutritional requirements and needs are vital. These require a multidisciplinary team, including a gastroenterologist, neurologist, dietitian, specialist nurse, and family members.
If needed, EN can provide the nutrients crucial to maintain a child’s health. This may require supplementation with fibre and can consist of both commercially available EN formulas, including real food-based formula and blenderised food, according to individual needs and tolerances.