Meeting Summary
Do flares of ulcerative colitis (UC) cause long-term bowel damage? Dr Sandborn opened the symposium by challenging the audience to think about their mindset regarding this question, as this may significantly influence therapeutic decisions. He then presented data showing the chronic, progressive nature of UC and argued that early appropriate therapy may slow disease progression. Dr D’Haens followed by describing the hurdles in achieving early and long-lasting remission in UC, like the delayed or suboptimal use of biologics. He also introduced strategies to overcome these hurdles, such as early intervention and treat-to-target. Dr Panaccione reviewed clinical trial and real-world data from current treatment options, specifically gut selective treatment, in the context of which outcomes patients value most.
Mission Assigned: Rapid Onset and Lasting Remission in Ulcerative Colitis
Doctor William J. Sandborn
Does Ulcerative Colitis Cause Long-Term Bowel Damage?
UC is still considered by many physicians as a benign disease that does not lead to long-term damage. To refute this, Dr Sandborn presented accumulating data on the progressive nature of UC. Using the example of the IBSEN study,1 a cohort of 423 patients with UC in Norway, diagnosed between 1990 and 1994 and systematically followed up for up to 10 years, he showed that an unfavourable disease course was observed in nearly 50% of patients: 37% followed a relapsing and remitting disease course, 6% had a chronic active disease course, and 1% experienced an increase in the intensity of their symptoms. He concluded that those patients who experienced an unfavourable disease course were, in his opinion, insufficiently treated.
Measures for the Progression of Ulcerative Colitis
The progression of UC has been measured in various studies against different outcomes, including proximal disease extension, colectomy, hospitalisation, colorectal cancer, and bowel damage. In a Swiss inflammatory bowel disease (IBD) cohort2 of 918 patients with UC, proximal disease extension occurred in around 15% of patients over a median disease duration of 9 years. When measuring disease progression by the need for surgery, data from the IBSEN study1 showed colectomy rates of around 10% in UC patients during the first 10 years following diagnosis. According to a review of various population-based cohort studies, hospitalisation occurs in around 50% of UC patients at some point during their disease course, which increases over time from 17–29% in the first year, to 29–54% within 5 years, and up to 66% in 10 years.3 A Danish nationwide cohort study of patients with UC demonstrated that the relative risk of colorectal cancer is significantly increased after 8–9 years of disease compared with the general population.4 Patients at significant risk are those who have failed to achieve endoscopic or histologic remission, those with extensive and long duration pancolonic disease, and those with concomitant primary sclerosing cholangitis.4
Although it is known that UC is progressive in nature, how to measure disease progression in individual patients remains an open question. In terms of morphological progression, unlike in Crohn’s disease (CD), UC is not typically accompanied by strictures and fistulising complications.5 In UC, mucosal appearance does not represent the total disease burden, since a disconnect occurs between endoscopic healing, resolution of acute inflammation, and the persistence of disabling symptoms.6 In summary, further research is needed to better understand the long-term functional consequences of UC, including comparing effective with ineffective inflammation control over long periods of time.5
Early Improvements: Better Long-Term Outcomes
Studies show that early endoscopic improvements can predict better long-term outcomes for patients with UC. A study of 157 newly diagnosed patients with UC receiving their first course of steroids, followed up over 5 years, demonstrated that patients in complete clinical and endoscopic remission after 3 months had a very low colectomy rate or need for immunosuppressive therapy, as well as a significantly reduced risk of relapse and hospitalisation compared with patients not achieving complete remission.7 Furthermore, a subanalysis of the infliximab ACT I and ACT II trials showed that a Week 8 Mayo endoscopy subscore of 0 or 1 (indicating mucosal healing) versus a score of 2 or 3 (indicating moderate or severe disease) predicted a significantly reduced rate of colectomy or need for rescue therapy.8
Early Intensive Treatment: Which Patients Can Profit?
Findings from CD studies show that early intensive treatment in some patients may be needed for optimal disease control. The CALM study9 demonstrated that early, intensive control with treatment in response to predefined targets including biomarkers (a treat-to-target approach), rather than only clinical symptoms, resulted in more patients achieving remission. Early intensive treatment should be personalised in UC and may not be necessary for all patients. It is therefore necessary to identify patients who may benefit from early intensive treatment. To guide the initiation of appropriate treatment in the right patients at the right time, we need a global evaluation of overall disease severity. The International Organisation for the Study of Inflammatory Bowel Disease (IOIBD) recently proposed a UC disease severity index providing a global disease severity evaluation to guide appropriate treatment initiation.10 The disease course, effects of the disease on the patient, and inflammatory burden are taken into account to assess the overall disease severity (Figure 1).10 A treatment strategy should also consider patients’ perceptions of their disease and treatment preferences.11

Figure 1: The International Organisation for the Study of Inflammatory Bowel Disease (IOIBD): Ulcerative colitis overall disease severity index.
CRP: C-reactive protein.
Adapted from Siegel et al.10
Mission Assessed: Challenges and Strategies to Achieve Early, Lasting Remission
Doctor Geert D’Haens
Why Do We Not Achieve Long-Lasting Remission in all Ulcerative Colitis Patients?
Not all patients with UC achieve early and lasting remission. This may be due to patients underestimating their disease activity, the inappropriate use of conventional therapy, and the delayed or suboptimal use of biologic therapy. A small survey comparing clinical or endoscopic remission with patient perceptions showed that patients with UC may underestimate their disease activity.12 A larger USA survey of 451 patients with UC and 300 gastroenterologists also showed discrepancies between patients’ responses and physicians’ assessments.13 The UC CARES study11 evaluated symptoms in 150 patients with moderate-to-severe UC receiving conventional therapies, and concluded unsatisfactory disease control (based on the number of daily stools and rectal bleeding) in over half of the patients. Patient quality of life was closely associated with unsatisfactory disease control, with overall work and activity impairment increasing with disease severity and even affecting patients in remission.12
An Inappropriate Treatment Choice?
The majority of patients with UC are still treated with conventional treatment (5-aminosalicyclic acid, corticosteroids, and immunosuppressants)14 and only a minority will eventually receive a biologic. Compared with CD, in patients with UC, physicians may be waiting too long to initiate biologic therapies; at the end of the first year following diagnosis, around 5% of patients with UC have received biologic therapy compared with 10–15% of patients with CD, according to epidemiologic data published in 2015.15
More recent data from an American insurance database showed the use of biologics between 2008 and 2016 in 6% of patients with UC (n=28,120) and 19% of patients with CD (n=16,260).16 Coupled with low uptake, biologics may also be used suboptimally. A USA insurance claims-based survey of 1,699 patients with UC receiving anti-tumour necrosis factor (TNF) treatment between 2005 and 2013 showed suboptimal use among 51% of patients within 6 months, and 91% of patients within 3 years. Drug switching, adding combination treatment, dose escalation, and discontinuations due to adverse events (AE) were used to identify suboptimal use of biologics.17
Strategies to Achieve Early, Lasting Remission
A number of strategies can be employed to achieve early, lasting remission (Figure 2);18 these include early intervention, treating to target, tight control monitoring, and individualised treatment.
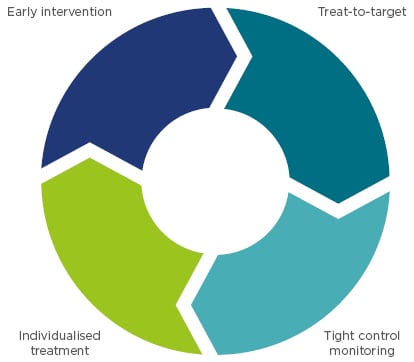
Figure 2: Clinical strategies associated with improved long-term outcomes.
Adapted from Colombel et al.18
Early intervention
A meta-analysis of 2,073 patients with UC showed that early mucosal healing was associated with improved long-term outcomes. Mucosal healing at the first endoscopic evaluation after initiation of therapy (compared with no mucosal healing) was 4.5-times more likely to lead to long-term clinical remission, and about 10-times more likely to lead to long-term steroid-free clinical remission.19
Further data supporting that early intervention may be associated with greater clinical response comes from the GEMINI 1 trial.20 Patients with UC with ≥1 but <3 years disease duration were more likely to have a clinical response to vedolizumab at Week 6 than those with a disease duration of ≥3 but <7 years (difference from placebo: 30.1% versus 18.5%).20
Treat-to-target
Treat-to-target is a concept used widely in the management of chronic diseases, such as rheumatoid arthritis,21 hypertension,22 and diabetes.23 The IOIBD concept, Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE), defines a composite of two targets for UC treatment: clinical remission, defined as resolution of rectal bleeding and normalisation of bowel habit, and endoscopic remission, defined as a Mayo endoscopic subscore of 0 or 1.24 Clinical remission should be assessed every 3 months until symptom resolution and every 6–12 months thereafter. Endoscopic outcome should be assessed 3–6 months after the start of therapy for a patient with symptoms.18,24
The feasibility of a treat-to-target strategy in clinical practice was tested among UC patients at the University of California, Oakland, California, USA, from 2011 to 2012. Proactive interventions, such as adjustments to medical therapy performed continuously over 80 weeks, resulted in 80% of patients reaching the target, compared with 30% of patients who had few or no interventions.25 Challenges with applying treat-to-target to clinical practice include a lack of data showing the effect on long-term disease modification, the evolution or inclusion of additional targets like histologic healing, potential overtreatment of low-risk patients, and proof of cost-effectiveness.18 Patients may be reluctant to follow their physicians’ advice and recommendations because they are fearful of complications, or they may be in denial about the severity of their disease. Patient education and a physician–patient shared decision is important when undertaking a treat-to-target approach.26
Tight control and monitoring
After achieving the target, tight control and monitoring are essential to obtain objective information on disease activity by monitoring symptoms, performing endoscopy, and measuring biomarkers to determine whether treatment adjustments are needed.27 A proposed algorithm for monitoring UC stratifies patients according to high or low risk of relapse and, in conjunction with biological markers, helps support the most appropriate treatment choice (Figure 3).28 Incorporating predictive tools into this algorithm, such as proteomics, genomics, or serologic markers that predict response to therapy, would enable further personalised treatment selection.18
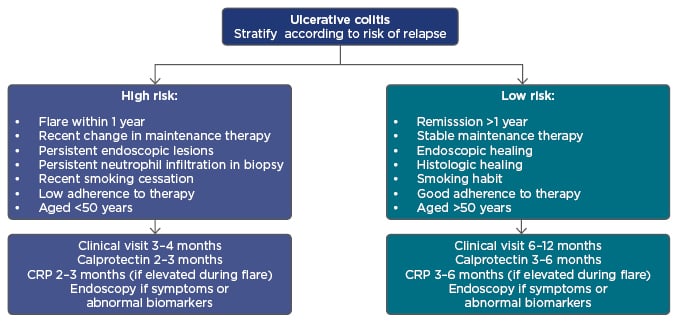
Figure 3: A proposed algorithm for individualising ulcerative colitis patient management.
CRP: C-reactive protein.
Adapted from Panes et al.28
Mission: Remission: Optimising Rapid Onset and Long-Term Outcomes with Gut-Selective Therapy
Doctor Remo Panaccione
Treatment Goals for Patients and Physicians
Physicians have different treatment goals to patients. For example, physicians aim to induce and maintain remission, and ultimately avoid complications, whereas patients want fast and sustained symptom relief with minimal side effects. Two commonly prescribed classes of biologic therapies approved for the treatment of moderate-to-severe UC are anti-TNF-α agents, which work systemically, and the gut-selective anti-α4β7 integrin, vedolizumab.29-32 Anti-TNF-α agents revolutionised treatment of UC with early remission rates reaching up to around 40% and Week 54 rates up to 35%.33-36 While generally safe, the TREAT registry of 5,394 patients with CD followed up over 5 years demonstrated an increased risk of serious infection in patients with moderate-to-severe disease and identified infliximab treatment as an independent risk factor.37 The European Crohn’s and Colitis Organisation (ECCO) consensus guidelines recommend particular care to be taken to consider serious infection as a complication of immunosuppressive therapy, including anti-TNF-α therapy.38
How Does Gut-Selective Treatment Meet the Goals?
A number of gut-selective biologics are in development.39 Vedolizumab is currently the only gut-selective anti-α4β7 integrin biologic with marketing authorisation for the treatment of moderate-to-severe UC. Rapid onset of action with vedolizumab has been reported in a post-hoc analysis of the GEMINI 1 trial data. Within 2 weeks of starting therapy, 30.8% of anti-TNF-α naïve patients with moderate-to-severe UC achieved complete resolution of rectal bleeding (versus 18.4% with placebo), and 44.6% of patients achieved a stool frequency subscore of ≤1 (versus 22.4% with placebo).40 The proportion of patients receiving vedolizumab who achieved these outcomes continued to increase between 2 and 6 weeks.40 The same trend is seen in the overall population, including patients with previous anti-TNF-α experience.40 Emerging evidence for early symptomatic improvement with the investigational anti-αEβ7 integrin, etrolizumab, showed rectal bleeding remission in approximately 20–25% of patients with anti-TNF-α refractory UC by Week 2 and about 40% of patients by Week 4.41
A further important endpoint has been analysed in another GEMINI 1 post-hoc analysis; mucosal healing, measured as a Mayo endoscopic subscore of ≤1, was achieved in almost half of the anti-TNF-α naïve patients as early as Week 6 (versus 25% with placebo), increasing to 60% by Week 52 (versus 24.1% with placebo). Improvements were also observed in anti-TNF-α failure patients but not to the same extent.42
These study data are complemented by real-world data from the US VICTORY Consortium;43 12-month real-world data showed that 77% of patients with UC receiving vedolizumab treatment achieved a Mayo endoscopic subscore of 0 or 1 at 12 months, with 53% of patients achieving a score of 0. Mucosal healing was similar among patients who were anti-TNF-α naïve or had one prior anti-TNF-α therapy.43 However, markedly fewer patients achieved either of these endpoints if they had previously received ≥2 anti-TNF-α therapies.44
Data on lasting clinical remission with vedolizumab can be derived from the GEMINI LTS cohort (see disclaimer). Of the patients who received vedolizumab induction and maintenance to Week 52 in GEMINI 1, followed by vedolizumab every 4 weeks in the open-label extension study, and had data available at around 5 years (n=63), 98% had a clinical response and 90% were in clinical remission.45
Vedolizumab Safety Profile
When considering long-term treatment, the safety profile of a drug is as important to the patient as its efficacy. The favourable safety and tolerability profile of vedolizumab has been confirmed by assessment of an integrated safety data analysis from >4,000 patient-years of vedolizumab exposure in IBD clinical trials.46,47 Low exposure-adjusted incidence rates for infections and no cases of progressive multifocal leukoencephalopathy have been reported.46-48 Real-world safety data from 2,857 vedolizumab-treated patients in 33 studies have been pooled and evaluated; the AE rate was consistent with that reported in the GEMINI programme, with low rates of serious AE, infections, and serious infections,48 reassuring patients and physicians that real-world clinical practice reflects outcomes recorded in clinical trials. As of August 2017, vedolizumab has 143,127 patient-years of post-marketing exposure worldwide.
In summary, treatment options are continuing to improve for IBD and UC, allowing physicians to better gauge patient values when choosing a therapy. Optimal use of biologic therapy will help patients achieve treatment targets of early and lasting remission. Systemic anti-TNF medications are effective in some patients and generally safe, but disease control can be limited33-36 and some safety issues remain.37,38
Early symptomatic improvement, long-lasting maintenance of clinical and endoscopic remission, and a favourable long-term safety profile make vedolizumab a suitable agent for both early and long-term treatment of UC.








