Meeting Summary
Inflammatory bowel disease (IBD) has a dramatic impact on patients and their families, as well as on society as a whole due to its significant economic impact around the world.1 Achieving the best outcomes for patients relies on early intervention, a treat-to-target (T2T) approach guided by a tight control (TC) strategy, and open dialogue with the patient allowing for individualisation of treatment.2 Dr Halfvarson discussed the current understanding of C-reactive protein (CRP) and faecal calprotectin (FC), both of which are useful biomarkers for the diagnosis, monitoring, adaptation of treatment, and prediction of relapse for IBD. Dr Bossuyt outlined that monitoring based on objective markers is very important in IBD because they can detect smouldering disease activity and also correlate with background inflammation.3,4 Dr Bossuyt also discussed new data emerging from the CALM study in Crohn’s disease (CD), concluding that using biomarkers as a TC strategy can be successful in IBD management because they reflect endoscopic outcomes independent of disease location and are the main drivers of treatment decisions during monitoring.5,6 Lastly, Prof Panaccione discussed the positive impact of the T2T approach on patient quality of life (QoL) and societal costs. In the CALM study, TC resulted in improved clinical outcomes, reduced CD-related hospitalisation, and improved QoL of patients.7–10 Furthermore, an extrapolated cost-effectiveness analysis of the CALM data over 2 years, taking into account indirect costs associated with improved work productivity, found that TC reduced overall societal costs and improved patient outcomes compared to clinical management (CM).11
Welcome and Introduction
Professor Remo Panaccione
In the 20th century, the industrialisation and Westernisation of many countries in Asia, the Middle East, and South America resulted in a surge in the incidence of IBD, making it a truly global disease.12–15 Onset of IBD is associated with significant disability and morbidity, usually occurring at a young age (15–40 years). Analysis of a population-based registry in northern France from 1988–2007 found that the median age at diagnosis was 26 years for CD (interquartile range: 20–38) and 35 years for ulcerative colitis (UC) (interquartile range: 26–47).16 This age range is generally when most people are economically and educationally productive,17 which therefore amplifies IBD’s economic impact worldwide.1
Aside from the disease and its symptoms, multiple factors can impact patients and their families, including depression, anxiety due to unpredictability, fatigue, chronic pain, lack of support, and financial burden.17 IBD also has an impact on regulators, payors, and society as a whole.1,18 Over time, the desired outcome for patients has evolved beyond symptom management; the target of IBD treatment should be the normalisation of QoL to a level the patient recognises from before their diagnosis.
The uptake of biologic therapies is still quite low, even in highly industrialised and developed countries such as the USA. In a recent analysis of the American MarketScan Insurance database, only 19% of patients with CD and 6% of patients with UC had received biologic therapy.19 Increased biologic uptake will likely have a large impact on the disease burden.
Achieving the best outcomes for patients begins with early intervention, followed by implementation of a T2T approach, including knowledge of the target and why it is important to both the clinician and the patient.2 A TC monitoring strategy is required to keep the target in sight, and open dialogue with the patient is paramount for understanding their values and individualising their treatment.2
The Story of Biomarkers So Far
Doctor Jonas Halfvarson
Regular use of endoscopy for monitoring in IBD is costly and invasive. A good surrogate marker of mucosal involvement would help to identify only those patients for whom a more invasive and expensive endoscopic procedure is warranted. CRP and FC are the two most well- characterised markers currently available.
STRIDE, a consensus programme by the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD), recommended in September 2015 that the composite target of the T2T approach for both CD and UC should be a combination of clinical or patient-reported outcome remission and endoscopic remission.20 At the time, before data from prospective trials such as CALM were available, biomarkers were not regarded as possible treatment targets but rather as adjunctive measures of inflammation.20 The more recent third European consensus recommendations on CD state that therapy and short-term follow-up can be guided, and relapse can be predicted by, levels of biomarkers such as serum CRP and FC.21 Furthermore, FC levels may help distinguish CD from irritable bowel syndrome and can act as a surrogate marker of mucosal healing.21 Both CRP and FC are therefore useful tools in the diagnosis, monitoring, and adaptation of treatment for IBD. Future research is required to define optimal cut-off values for diagnosis and follow-up, understand intraindividual variations of FC, and determine optimal timing of stool sampling and frequency of measurement of FC.
A meta-analysis of 13 studies including 744 patients with UC and 727 patients with CD found that if a low FC cut-off such as 50 µg/g is selected, then good sensitivity (92%) but only modest specificity (60%) is achieved.22 If the threshold is increased to 250 µg/g, sensitivity is reduced (80%) but specificity increases (82%).22 Given that the diagnostic accuracy of available FC assays differs,23 when these data are merged and reviewed it is difficult to confidently arrive at an ideal threshold value. Intraindividual variability also needs to be taken into account; indeed, great variability in FC concentration has been observed even during a single day.24 It is unlikely that one single cut-off can be applied to all the various situations in which FC is thought to play a role and so it is probable that there will be different thresholds depending on the factors present.
Decreasing FC concentration has been found to correlate with clinical response and mucosal healing. In an early study, FC levels in a single patient with a UC flare began to decrease even after 1 week of treatment (prednisolone 80 mg and 5-aminosalicylic acid 3 g/day) and almost normalised prior to the patient achieving clinical remission.25 Røseth et al.25 also noted large reductions in median FC concentration when patients progressed from active disease to clinical remission. Endoscopic assessment found that 97.8% of patients (44 out of 45) had complete mucosal healing and 84.4% (38 out of 45) had no histological activity. Furthermore, in a more recent retrospective chart review of 68 patients with UC, FC levels significantly correlated with endoscopic extent, mucosal healing, and histological activity.26 An FC ≤60 μg/g robustly predicted deep remission (area under the curve: 0.92; sensitivity: 86%; specificity: 87%), indicating that FC can be used in a T2T approach in patients with UC.26
Numerous studies in patients with quiescent disease have demonstrated that increased FC levels can predict disease relapse within 12 months, particularly in patients with UC.27–31 However, these measurements represented only snapshots of this chronic disease; biomarkers should be measured continually over time, as was done in the prospective STORI trial.32 In 115 patients with CD, infliximab treatment was stopped and patients were followed for at least 1 year. In multivariate analysis, FC ≥300 µg/g was significantly associated with time to relapse (p=0.04). Also, a higher proportion of patients with an FC ≥300 µg/g relapsed (15 out of 21; 71.4%) compared with those with an FC <300 µg/g (25 out of 64; 39.1%; p=0.0002). Similarly, in a prospective study of adult patients with UC in clinical remission receiving ongoing infliximab maintenance therapy (n=87), FC levels remained <40 µg/g in patients with sustained deep remission, while patients experiencing flares had significantly higher FC levels 3 months before flare.33 Moreover, two consecutive FC measurements of >300 µg/kg within a 1-month period predicted the risk of clinical flare with a very high specificity (100%) and modest sensitivity (61.5%).33 Similar work has been undertaken to adapt this approach to a broader population of patients in clinical remission in the outpatient clinic. In a prospective study of 49 patients with CD and 55 patients with UC, in whom FC was measured every 3 months, doubling of FC concentration was associated with a 101% increased risk of relapse (hazard ratio [HR]: 2.01; 95% confidence interval [CI]: 1.53–2.65; p<0.001).34 Moreover, relative risk of relapse attenuated with time (HR: 0.80; 95% CI: 0.75–0.86; p<0.001), decreasing by 20% per 3-month period.34 Therefore, longitudinal monitoring of FC can aid evaluation of relapse risk in IBD. Lastly, the development of remote (at-home) tests for patients can facilitate the measurement of FC for routine disease monitoring. This type of eHealth initiative is more convenient for patients and has been identified as important by the European Crohn’s and Colitis Organisation (ECCO).35
Can We Trust Biomarkers in Inflammatory Bowel Disease Management?
Doctor Peter Bossuyt
Currently, the available evidence indicating that biomarkers can predict relapse and act as surrogate markers for endoscopy is largely retrospective and/or circumstantial. CALM,7 an open-label, randomised, controlled, Phase III study conducted in Europe and Canada, was the first trial to prospectively show that a TC algorithm of monitoring symptoms and biomarkers leading to a T2T strategy is successful over time and results in better outcomes. Patients with moderate-to-severe active CD received induction therapy with prednisone for up to 8 weeks and were subsequently randomised 1:1 to CM (n=122; escalation based on CD Activity Index [CDAI] and prednisone use) or TC (n=122; escalation based on CDAI, prednisone use, CRP, and FC). At each of the four clinical assessments, treatment could be escalated if needed based on the different criteria used in each arm. The investigators were blinded for the biomarker results during the trial. The intensification sequence was no treatment, adalimumab 40 mg every other week, adalimumab 40 mg weekly, and, finally, adalimumab 40 mg weekly plus daily azathioprine (2.0–2.5 mg/kg). Options were available for rescue if escalation was needed before the next study visit and for treatment de-escalation once the success criteria were met. Overall, 76% (93 out of 122) of CM patients and 74% (90 out of 122) of TC patients completed the study.
The results from CALM have been presented previously.7 In summary, mucosal healing (CD Endoscopic Index of Severity <4) without deep ulceration was significantly higher with TC than with CM (45.9% versus 30.3%; p=0.01; Figure 1). TC also achieved significantly higher rates of deep remission (36.9% versus 23.0%; p=0.014), biological remission (29.5% versus 15.6%; p=0.006), and mucosal healing (45.9% versus 30.3%; p=0.01) compared with CM.
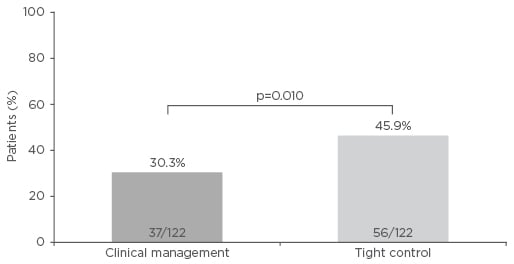
Figure 1: CALM primary endpoint (mucosal healing [Crohn’s Disease Endoscopic Index of Severity <4] and no deep ulcerations) at 48 weeks after randomisation.
Adapted from Colombel et al.7
Additionally, rates of mucosal healing in all segments, complete endoscopic remission, and endoscopic response were higher in the TC group than in the CM group but not significantly. These endoscopic and biomarker outcomes were reflected in the clinical outcome during the trial. At every time point, the rate of steroid-free clinical remission was significantly higher with TC compared with CM (Figure 2).
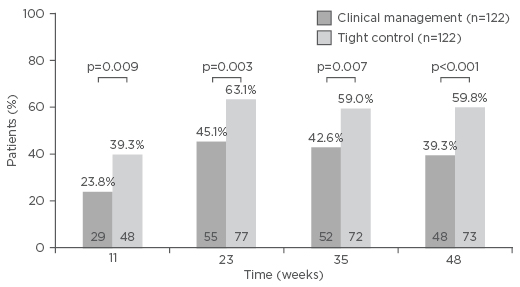
Figure 2: Steroid-free remission (Crohn’s Disease Activity Index <150 and no corticosteroids for ≥8 weeks) during the post-randomisation period of the CALM study.
Adapted from Colombel et al.7
Monitoring based on objective markers is very important in IBD since smouldering disease activity can affect long-term outcomes. In an observational study of 185 patients with CD in clinical remission, patients with elevated CRP demonstrated worsening of disease based on a change in Lémann Index compared with patients with normal CRP levels (65% versus 36%; p<0.0001).3 Elevated CRP was associated with a 7-fold higher likelihood (odds ratio: 6.93; p<0.0001) of bowel damage over time.3 Likewise, analysis of data from the Phase III, randomised, controlled POCER study demonstrated a good correlation between FC and background inflammation. It was observed that the Rutgeerts score rose over time with increasing FC level, even if the patient was in clinical remission.4
Monitoring strategies must be accompanied by appropriate intervention based on findings to improve outcomes. A retrospective study of 67 patients with CD undergoing monitoring by endoscopy found that timely assessment (<26 weeks between endoscopic procedures; HR: 2.35; 95% CI: 1.15–4.97; p=0.035) and adjustments to medical therapy when mucosal healing was not reached (T2T; HR: 4.28; 95% CI: 1.9–11.5; p=0.0003) increased the likelihood of mucosal healing.36 CALM has now shown that a TC algorithm of monitoring symptoms and biomarkers optimises this strategy.7
In further analysis of CALM data, rates of mucosal healing with no deep ulceration were shown to be associated with biomarker levels at Week 48, with odds ratios of achieving the endpoint of 4.5 for CRP <5 mg/L (95% CI: 2.3–8.7) and 18.4 for FC <250 µg/g (95% CI: 7.7–44.0).5 When the two biomarkers were combined, an even stronger association was observed, although this was mainly driven by FC <250 µg/g.5 A high association between biomarkers and mucosal healing without deep ulceration was observed irrespective of disease location, even for isolated ileal disease. This was most evident with FC <250 µg/g but less so for CRP <5 mg/L.5 Lastly, it was assessed whether biomarker findings or clinical assessment of patients was the predominant driver of treatment decisions. In the TC group, there was a progressive shift from multiple drivers (CDAI ≥150, CRP ≥5 mg/L, FCP ≥250 μg/g, and prednisone use) to one or two drivers of the treatment decision. At Week 35, only one (15.8%) or two (4.2%) failure criteria were driving the treatment decision.6 At every time point, it was found that for most patients, FC or CRP for one criterion and FC plus CRP for two criteria were driving the treatment decision, rather than clinical assessment of the patient (using CDAI) or prednisone use.6
In summary, biomarkers are reliable tools in IBD management because they have been demonstrated to be the main drivers of the treatment decision during monitoring and reflect endoscopic outcomes independent of disease location in CD. The best outcome for a patient relies on a timely T2T approach guided by monitoring of objective biomarkers. For this, it is key to have a strict treatment plan comprising four important steps when starting treatment in patients with active CD. Firstly, a treatment target needs to be determined and currently mucosal healing is the optimum target. Next, before the start of treatment, a baseline assessment of the biomarkers is mandatory to identify the optimal monitoring tool, and then a plan with strict monitoring visits needs to be discussed with the patient upfront. Lastly, if the biomarker or clinical monitoring still indicates active disease, the treatment must be adapted accordingly. This approach can ensure a good journey to reaching the target, ultimately resulting in successful treatment.
Reducing Patient Burden and Societal Cost
Professor Remo Panaccione
A Canadian literature review found that patients with IBD had lower generic QoL scores (Short Form-36) compared with the general population.37 Regarding utility scores, those with moderate-to-severe disease had very low mean scores (0.45), indicating significant impairment to QoL, while patients with mild disease scored a mean of 0.68, a level of impairment typical of many chronic diseases. Patients in remission had a mean score of 0.77, which is also somewhat below the population average of 0.85.37 These findings highlight patients’ needs in normalisation of QoL, which can be achieved through resolution of symptoms and inflammation as well as prevention of disease progression.
One way forward is to develop treatment algorithms, such as the early optimisation algorithm assessed in the open-label, controlled REACT trial.38 Practices in Belgium and Canada were randomised (1:1) to treat patients with either early combined immunosuppression (ECI) with a tumour necrosis factor antagonist and an antimetabolite, and received training in the treatment algorithm, or to treat with conventional management. Patients were followed for 2 years and reviewed every 12 weeks; if they had not achieved clinical remission, their treatment was escalated. There were significant differences in favour of ECI over 24 months for patient-level time to surgery (p=0.0314), serious disease-related complications (p<0.001), and composite major adverse outcomes (p<0.001). The difference in time to hospitalisation also favoured ECI but was not significant. Therefore, despite ECI not being more effective for controlling symptoms of CD than conventional management, the risk of major adverse outcomes was lower. REACT239 is an ongoing study assessing if an enhanced treatment algorithm incorporating early combination therapy and endoscopic evaluation can improve the management of patients with CD in comparison to the conventional step care algorithm. The primary endpoint is risk of CD-related complications at 1 year.
Improvement in clinical outcomes and QoL has also been observed under intense monitoring disease management. In a separate analysis of the CALM study,8,9 early significant separation in time to CD flare (increase in CDAI ≥70 points from 1 week prior to randomisation, or early randomisation and CDAI >220) was observed between the CM and TC groups (HR: 0.4; 95% CI: 0.2–0.8; p=0.012). Furthermore, significantly fewer CD-related hospitalisations after randomisation occurred in the TC group than in the CM group (p=0.021; Figure 3). The proportion of patients who experienced CD-related hospitalisations or serious complications was lower in the TC group (14.8%) than in the CM group (20.5%) but not statistically significant (p=0.240). The time-to-event curves began to separate at Week 15 after remission but again the difference was not statistically significant (HR: 0.7; 95% CI: 0.4–1.3; p=0.249). Based on these results, it is likely that statistical significance of separation will be achieved with further follow-up. When investigating patients’ QoL, another analysis of CALM found that, at all time points, more patients in the TC group were in IBD questionnaire remission (≥170 points) and had an IBD questionnaire response (mean change score ≥16 points) compared with the CM group.10 Overall, TC is associated with lower rates of CD flare, hospitalisation, and serious complication, as well as improved QoL compared with CM.
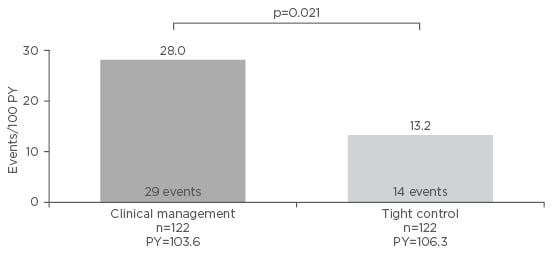
Figure 3: Rate of Crohn’s disease-related hospitalisations after randomisation in the CALM study.
PY: patient-years.
Adapted from Colombel et al.8 and Colombel et al.9
Intense monitoring, optimisation of treatment, and dose escalation can be expensive; however, costs should be evaluated in light of the associated clinical benefits. In an economic evaluation using 48-week data from the CALM study, initially presented at the 2017 United European Gastroenterology (UEG) Week,40 the incremental cost-effectiveness ratio (ICER) of TC was found to be £20,913 per quality-adjusted life year (QALY). TC is considered cost-effective as the ICER is below the £30,000 threshold based on guidelines by the National Institute for Health and Care Excellence (NICE).41 This study mainly focussed on direct medical costs in a 48-week time horizon. Presented during the 2018 ECCO Congress was an updated economic model, accounting for both the direct and indirect costs associated with work productivity over a 2-year timeframe using projection data from CALM.11 Over the 2 years, total direct medical costs were £25,808 and £24,939 for TC and CM, respectively. The difference in QALY was found to be 0.09 higher for TC and resulted in an ICER of £10,102 per QALY gained. When including costs associated with reduction in absenteeism, total costs over 2 years were lower with TC (£16,480) than with CM (£18,733), resulting in a difference of -£2,253. TC thus became dominant (i.e., less costly and more beneficial) compared to CM from a societal perspective.
In conclusion, CALM showed that TC is a cost-effective approach associated with improved clinical outcomes, reduced CD-related hospitalisation, and improved patient QoL in the long term. The economic value of TC is more apparent when considering the evaluation from a societal perspective, taking into account indirect costs.11 The increased costs of drugs resulting from treatment escalation were offset by a reduction in direct and indirect medical costs.
Conclusion
Tightly controlled monitoring of patients with IBD and analysing data to guide subsequent therapeutic action is essential to improve patient outcomes and prevent future flares. The therapeutic goals of physicians, patients, and wider society should align through the T2T approach to achieve optimal IBD outcomes and reduce disease burden. An open dialogue with the patient is vital to develop acceptable strategies for baseline assessment, determination of the therapeutic goal, and regular monitoring.








