Meeting Summary
Crohn’s disease (CD) is a chronic, progressive, relapsing-remitting disorder characterised by periods of inflammatory activity occurring most commonly in the terminal ileum and colon, resulting in worsening bowel damage and increasing disability, which in turn are associated with significant impairment in quality of life (QoL). The recognition of CD as a progressive disease has shifted the goal of treatment from symptom management towards a focus on slowing disease progression, with the aim of reducing subsequent disability and mitigating impacts on QoL. This symposium focusses on understanding the advantages and limitations of current management strategies. It addresses the full spectrum of the complexity of CD, ranging from biologic therapy for moderately-to-severely active luminal CD, to new treatment options for complex perianal fistula based on innovative stem cell approaches.
Evolving Approaches for Managing Progressive Crohn’s Disease
Professor Jean-Frédéric Colombel
Since the early 2000s, it has become increasingly evident that CD is not just a relapsing-remitting disease. It is a progressive, destructive disease leading to an accumulation of bowel damage and serious, potentially disabling complications, such as strictures, abscesses, and fistulas.1 As a result, it is essential to optimise not only treatment choices but the overall approach to the management of this disease.
Prof Colombel shared his experience working at the Mount Sinai Hospital, New York City, New York, USA in a programme called Gaining Resilience Through Transitions (GRITT), managing inflammatory bowel disease (IBD) patients in a multidisciplinary team. This initiative has enabled the provision of high-quality care for patients using telemedicine and digital health, while simultaneously decreasing costs and time-burden for treating physicians. Based on this experience, Prof Colombel proposed solutions for optimal use of therapies in Crohn’s disease summarised in six key points:
- The right concept.
- The right time.
- The right drug.
- The right target.
- The right monitoring.
- The right team.
The first key point is the right concept. A key treatment goal for patients with CD should focus on slowing disease progression and damage, as well as on acute symptomatic improvement and reduction of inflammation. To do so, it is crucial to implement treatment strategies at the right time. Interventions should be initiated during the early stages of CD, wherein there is thought to be a ‘window of opportunity’ during which treatment might be able to alter the course of the disease to reduce eventual bowel damage and disability.2 Several trials evaluating TNF antagonists suggest that the earlier a patient with CD is initiated with a biologic, the better the efficacy outcomes in terms of remission and response.3-10
Choosing the right drug for a patient is also critical. Treatments can vary from nutritional therapy, conventional corticosteroids and immunosuppressants, biologic medications, and stem cell therapy to surgery. Due to the heterogeneity of CD, the most suitable intervention or combination of interventions should be selected based on multiple factors. These include disease duration and severity, long-term risk of progression, risk:benefit ratio of the therapy, and comorbidities and complications, as well as patient preference.
The International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) supports the next important point: the right target. They provide evidence and consensus-based recommendations for selecting the goals for ‘treat to target’ (T2T) strategies in patients with CD.11 The IOIBD concluded that targeting clinical resolution of symptoms alone is insufficient and does not appear to significantly alter the natural course. Mucosal healing or endoscopic remission, however, provides an objective assessment of inflammation and has been shown to be associated with better outcomes in cohort studies and randomised controlled trials.12–17 Therefore, the treatment target should be a composite endpoint involving clinical/PRO remission (defined as a resolution of abdominal pain and normalisation of bowel habit, assessed at a minimum of 3 months during active disease) and endoscopic remission (defined as resolution of ulceration, assessed at 6–9-month intervals during the active Phase).11
Once an intervention is in place, the right monitoring, by evaluating symptoms, inflammatory biomarkers, and endoscopy, is required to ensure tight disease control.11,18 This allows for immediate action when the patient fails to respond to a treatment or becomes unresponsive after initial treatment success. Therapeutic drug monitoring provides the opportunity to ensure the patient is receiving the right drug at the right dosage.
Last, but not least, is the importance of the right team. A multidisciplinary healthcare team (MDT) infrastructure has been shown to improve outcomes for patients with CD. The team may include gastroenterologists, colorectal surgeons, nurses, radiologists, dieticians/nutritionists, pathologists, pharmacists, hospital management, and researchers, as well as other functions as appropriate. Depending on the stage of the patient’s disease, different members of the MDT will be key.
Maximising Outcomes with Early Effective Pharmacologic Treatment of Crohn’s Disease
Professor Stefan Schreiber
Prof Schreiber further emphasised the importance of initiating treatment at the right time for patients with CD. Indeed, a growing body of evidence supports the concept that initiation of treatment with disease modifying anti-IBD drugs (DMAID) during the ‘window of opportunity’ of early-stage CD (up to approximately 18 months from diagnosis) may change the natural progression of the disease. This, in turn, may then reduce the chances of irreversible damage and associated disability (Figure 1).19 However, therapeutic goals during early-stage CD differ considerably compared with treatment goals for late-stage disease.
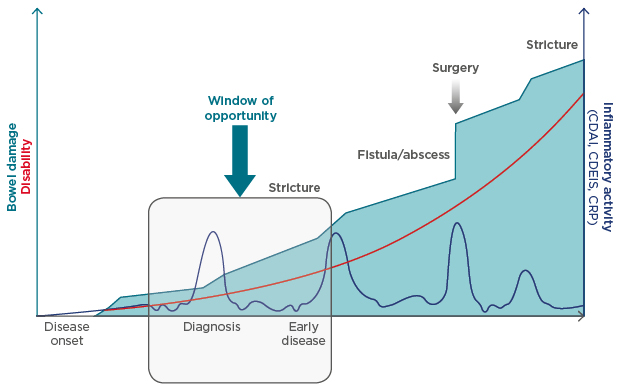
Figure 1: Early intervention and the progression of Crohn’s disease.
Current evidence suggests that there may be a window of opportunity for early intervention with DMAID to change the natural progression of Crohn’s disease and to prevent or reduce future complications.
CDAI: Crohn’s disease activity index; CDEIS: Crohn’s disease endoscopic index of severity; CRP: C reactive protein; DMAID: disease modifying anti inflammatory bowel disease drugs.
Adapted from Pariente et al.1 and Colombel et al.2
During early-stage CD, an appropriate treatment goal is to attain a complete absence of symptoms, absence of complications or disability, achieving normal QoL, and ultimately slowing disease progression. However, these goals are not realistic in late stage disease, where the focus should be on stabilisation of non-inflammatory symptoms, absence of progression in bowel damage or disability, and improvement in QoL.20
Choosing the Appropriate Pharmacologic Treatment for Individual Patients
As previously highlighted, early interventions in appropriate patients are necessary, and, therefore, individualising treatment strategies for patients with CD is recommended. This means that patients with milder CD can be treated differently to those with more aggressive, severe CD. In mild CD, there is a space to consider the ‘step up’ approach to avoid unnecessary immunosuppression and adverse events (AE). Conversely, in patients with more aggressive and rapidly progressing disease, a ‘top down’ approach using intensive therapy at an earlier stage to avoid future complications may be considered.19
To aid physicians with rapid decision making, identification of the patients in whom early intensive therapy is appropriate is critical. Recognising the prognostic factors for disease progression helps to identify those patients. As part of the IBD Ahead 2014 educational programme, a panel of IBD experts from 32 countries worldwide identified several prognostic factors for disease progression in CD, including ileal disease location, upper gastrointestinal involvement and extraintestinal manifestations, younger age or perianal disease at diagnosis, smoking, endoscopic severity, serologic reactivity to microbial antigens, and certain genetic mutations (e.g., NOD2).21 These factors may represent a first step in stratifying patients into low or high-risk groups to determine appropriate treatments and therapeutic targets.
Early Use of Biologic Therapy is Associated with Improved Outcomes
Biologic medicines, including adalimumab and vedolizumab, have provided much needed alternatives to steroid-based therapy in patients with CD. Moreover, early use of biological therapies is associated with improved treatment outcomes of clinical remission and mucosal healing. In a real-world observational study of 650 patients, those who received early vedolizumab (≤2 years from diagnosis) were associated with better clinical remission, steroid-free remission, and mucosal healing compared with those who received late vedolizumab (>2 years from diagnosis).22 Another real-world study of 122 patients demonstrated significantly fewer CD-related flares in patients receiving early vedolizumab compared with those who received late vedolizumab.23
The REACT study,24 an open-label, cluster randomised clinical trial, evaluated the strategy of early intervention with combined immunosuppression (ECI) with adalimumab therapy, versus conventional management. For the ECI strategy, disease activity was assessed every 12 weeks and treatment was modified if necessary. Conventional management was a step-care sequential algorithm according to the usual practice of the physicians. Stating the case for fast decision making, the ECI strategy with regular assessment and intervention resulted in reduced risk of hospitalisation, surgery, or serious disease-related complications versus conventional management.24
Although there is a consistent trend towards the increased use of biologics over time, many patients with CD still do not receive them. For example, in 2014, only around one-third of CD patients in Norway had ever received a biologic for treatment of CD.25 An important contributing factor to the limited use of biologics may be safety concerns. A survey of patients with IBD evaluated the attributes of biologic treatment (i.e., mechanism of action, mode of administration, efficacy, and side effect profile) that drive treatment decision making. Patients with CD were found to prioritise safety attributes over all other attributes, demonstrating the importance of minimising side effects.26 A comparative safety study in the real-world setting using matched propensity scores has shown vedolizumab to be associated with less serious infections and serious AE compared to TNF antagonist treatment.27
Multidisciplinary Care Improves Outcomes
Prof Schreiber also emphasised that early and appropriate therapy should be delivered by a structured and collaborating MDT, putting the patient at the centre to ensure optimal treatment is provided. Patients with early-stage CD are often treated in a community setting,28 but this should not prevent access to a MDT.
Fistulising Crohn’s Disease: Current Treatment Challenges
Professor Gert van Assche
While the other speakers focussed on the beneficial effects of early intervention, Prof van Assche concentrated on a typical late-stage Crohn’s complication. Fistulas are one of the most frequent and disabling complications of CD. A population-based cohort study reported that the cumulative incidence of fistulas rises steadily from approximately 20% within the first year of diagnosis to 50% after 20 years, with almost half of these cases having perianal manifestations.29 A perianal fistula is the initial disease presentation in approximately 10% of patients with CD and may precede the manifestation of intestinal disease by several years.30 Delays in diagnosis of CD in the presence of fistulas is a significant problem, which may be attributed to late referral of patients to a gastroenterologist or to a centre with IBD expertise.
Perianal fistulas negatively impact on patients’ QoL and are often persistent despite treatment.31,32 The presence of fistulas is known to predict a disabling disease course33 and half of complex cases require complicated surgical interventions, such as stomas, resection, and proctectomy.34 Physical symptoms, such as anal pain and discomfort, and restriction of daily and sexual activities, are important concerns for patients.32,35 Moreover, patients are reluctant to talk about the impact of perianal disease on their daily lives and patient reporting of the burden is influenced by intercultural differences. As such, there is a substantial unmet medical need for improved treatment options.
Perianal Fistulising Crohn’s Disease is a Complicated Treatment Challenge
The short-term goals for treatment of perianal fistulas in CD are to drain abscesses and reduce symptoms. In the longer-term, aims are to halt any discharge and ensure healing, improve QoL, preserve continence, and avoid proctectomy.36 Overall, treatment should be individualised according to the type of fistula, degree of rectal inflammation, and severity of symptoms.37
Several treatment options are available, and a ‘step-up’ algorithm is generally used (Figure 2). After initial treatment with antibiotics and thiopurines, which are useful adjunctive treatments despite their limited and unproven efficacy when used alone, biologic therapies are often used as the next option. Biologics provide effective short-term remission of fistulas in approximately 28–55% of patients.7,38 However, >50% of these patients are likely to relapse within 1 year39 or after cessation of therapy.40 In one study, MRI-based disease activity scores demonstrated that infliximab had a major impact on perianal fistulas in the short and medium-term. Of note, efficacy was not maintained long-term, and at 95 weeks there was no significant difference in MRI score versus baseline.41
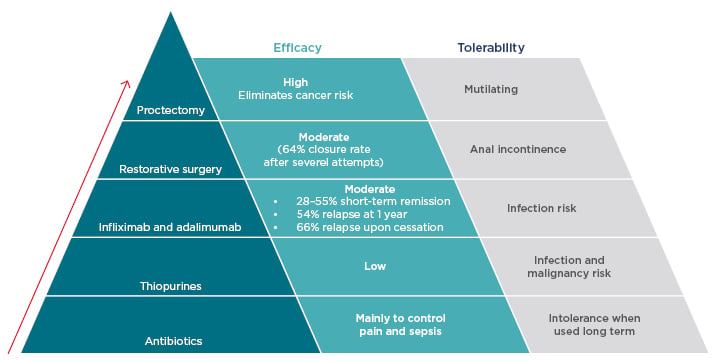
Figure 2: Step up treatment algorithm for perianal Crohn’s disease.
The currently accepted treatment algorithm for perianal Crohn’s disease is based on the concept of ‘step-up’ therapy, progressing antibiotics through biologic therapy to surgical techniques.
After exhaustion of drug-based therapeutic options, surgical procedures become necessary but are poorly tolerated. Restorative surgery can be successful for some patients, but the fistula closure rate is only moderate42 and there is a risk of anal incontinence. Proctectomy is recommended only as a last resort36 as, although highly effective, it is a mutilating procedure with considerable risks, including pelvic nerve damage, presacral abscesses, and delayed perineal wound healing. Unfortunately, owing to the limitations of other treatment options, proctectomy remains a reality faced by many patients with CD.36
By reviewing the evidence of pharmacological therapy in conjunction with surgical treatments, the paucity of effective treatments for patients with fistulising CD is apparent and represents a significant unmet need. A novel and innovative technique using stem cell therapy to treat complex perianal fistulas has received marketing authorisation in Europe. This therapy, darvadstrocel (Alofisel®, Takeda Pharma A/S, Taastrup, Denmark), previously Cx601, is available for patients with non-active/mildly active luminal CD, who have shown an inadequate response to at least one conventional or biologic therapy and looks promising for this hugely underserved population of patients.
Transforming Treatment of Fistulising Crohn’s Disease: New Stem-Cell Based Approaches
Professor Damián García-Olmo
Prof García-Olmo further accentuated that patients with fistulising CD are highly challenging to treat and that there is a lack of effective treatments. Patients with fistulising CD are known to be particularly refractory to conventional medical strategies of antibiotics, immunomodulators, and TNF antagonists. Pharmacological therapies serve to provide a degree of symptom improvement often in the short-term, but long-term complete healing is rare. Ultimately, surgical procedures become inevitable after repeated relapses. A number of surgical options are available for the treatment of fistulas, including obturation (fibrin glue and/or fistula plugs), chronic seton placement, endorectal mucosal advancement or local perineal flaps, sphincteroplasty, and ligation on the intersphincteric tract (LIFT).43,44 However, each of these options is associated with at least one important limitation, such as a medium or high rate of fistula recurrence, anal incontinence, postoperative pain, and/or the technical difficulty of performing the procedure. Therefore, there is a clear need for effective late-stage treatments or procedures that are not associated with any of these issues.
Stem Cell Therapies Offer the Potential for Improved Fistula Healing
The essential conundrum of therapy for perianal fistulas is the difficulty of inducing wound healing. The anti-inflammatory and immunomodulatory properties of mesenchymal stem cells offer the potential to induce healing of the fistula without the need for gastrointestinal tract surgery.45 Clinical proof of concept was first demonstrated in 2003 using autologous adipose-derived stem cells (ASC) for the successful treatment of a young woman with a recurrent rectovaginal CD fistula unresponsive to medical treatment.46 Further Phase I and II studies presented positive data for autologous ASC for the treatment of complex perianal fistulas in patients with CD, with induction of healing observed in 70–82% of patients and no AE considered to be related to treatment with ASC.47-49
In clinical practice, the use of allogeneic stem cells is preferable to autologous stem cells because it avoids the need to collect primary cells from the patient. The feasibility of this option could provide an ‘off-the-shelf’ treatment that would be accessible to more patients, more affordable, and be available for more rapid administration.50 In recent years, investigations into the use of stem cell therapy in CD has focussed on the use of allogeneic stem cells with promising results.
Allogeneic Adipose-Derived Stem Cells are a Promising Option for the Treatment of Complex Fistulas
ADMIRE51 was a Phase III, randomised, double-blind, placebo-controlled trial that assessed the efficacy and safety of allogeneic ASC (darvadstrocel) for treatment-refractory complex perianal fistulas in adult patients with CD. Patients received standard of care plus either darvadstrocel or placebo. The primary endpoint was combined remission at Week 24, defined as the closure of all treated external openings that were drained at baseline (clinical remission) and absence of collections >2 cm of the treated perianal fistulas, confirmed by blinded central MRI.51
The proportion of patients who achieved combined remission was significantly higher with darvadstrocel treatment compared with placebo at Week 24 (51.5% versus 35.6%; p=0.021) and was maintained at Week 52 (56.3% versus 38.6%; p=0.010) (Figure 3).51,52 Median time to clinical remission occurred at around 7 weeks in the darvadstrocel group and 15 weeks in the placebo group, indicating that darvadstrocel treatment also resulted in more rapid healing than the current standard of care.53 Relapse rates at Week 52 were lower with darvadstrocel compared with placebo (25.0% versus 44.1% [95% confidence interval: -39.5–1.3]),53 which is particularly encouraging given that approximately half of patients treated with antiTNF therapy for perianal fistulas experience a recurrence.54 The safety data at Week 52 confirmed a favourable tolerability profile for darvadstrocel, which was maintained over a prolonged period and the frequency and type of AE was similar in the two groups.52
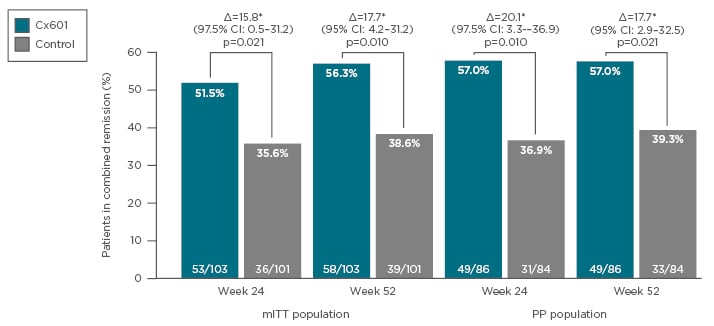
Figure 3: Combined remission of perianal fistulas at Week 24 and Week 52 following treatment with Cx601 or placebo.
CI: Confidence interval; mITT: modified intention to treat; PP: per protocol.
Adapted from Panés et al.51 and Panés et al.52
These findings have potentially significant implications for the treatment of perianal fistulas in clinical practice. The sustained response observed with darvadstrocel could reduce the need for major surgical interventions, resulting in lower risk of incontinence, and reduce the need for systemic immunosuppression, providing a more benign tolerability profile. Stem cell therapy is therefore a pivotal step to addressing the unmet need for a safe and effective treatment of complex perianal fistulas in CD.
Concluding Remarks
Current evidence suggests that there may be a ‘window of opportunity’ for earlier, timely intervention with DMAID to change the natural progression of CD and prevent or reduce future debilitating complications. Evidence from clinical trials suggests that the use of vedolizumab in early-stage disease is associated with better clinical remission and mucosal healing compared with late-stage disease. In later stages of the disease, fistulising CD continues to present a particularly complex clinical challenge, with current treatment options limited by low success rates, tolerability issues, and the risks associated with surgical procedures. Alofisel stem cell therapy is a promising new option for fistulising CD, which addresses many of the pitfalls of current treatment options and is associated with a growing body of clinical evidence demonstrating efficacy and safety. Care for patients with CD should involve a well-aligned MDT to ensure effective management of the diverse burden of disease, supported by clear patient communication, at all stages.








